23 4.4 Oxidation-Reduction Reactions
Learning Objectives
By the end of this section, you will be able to:
- Define oxidation and reduction.
- Use a chart to assign oxidation numbers to atoms in simple compounds.
- Recognize oxidation-reduction reactions.
- Recognize combustion and single replacement reactions.
Redox Reactions
Earth’s atmosphere contains about 20% molecular oxygen, O2, a chemically reactive gas that plays an essential role in the metabolism of aerobic organisms and in many environmental processes that shape the world. The term oxidation was originally used to describe chemical reactions involving O2, but its meaning has evolved to refer to a broad and important reaction class known as oxidation-reduction (redox) reactions.
The most easily identified redox reactions involve the exchange of electrons between reactants. A reactant that loses, or gives up electrons during such a reaction is said to be oxidized as it is converted to product. A reactant that gains, or accepts electrons during the reaction is said to be reduced during the reaction.
What strange, counter-intuitive language this is! It may be helpful to think about the charge on a species (atom, ion or formula) being reduced during the reaction, as electrons with their negative charges are accepted.
Some redox reactions involve the transfer of electrons between reactant species to yield ionic products, such as the reaction between sodium and chlorine to yield sodium chloride:
What happens in such a reaction can be made more clear by considering each individual reactant in the form of an equation called a half-reaction:
[latex]\text{Cl}_2(g) + 2\text{e}^{-} \longrightarrow 2\text{Cl}^{-}(s)[/latex]
These equations show that Na atoms lose electrons while Cl atoms (in the Cl2 molecule) gain electrons, the “s” subscripts for the resulting ions signifying they are present in the form of a solid ionic compound. For redox reactions of this sort, the loss and gain of electrons define the complementary processes that occur:
In this reaction, then, sodium is oxidized and chlorine undergoes reduction.
Some redox processes, however, do not involve the actual transfer of electrons. Consider, for example, a reaction similar to the one yielding NaCl:
- The oxidation number of an atom in an elemental substance is zero.
- The oxidation number of a monatomic ion is equal to the ion’s charge.
- Oxidation numbers for common non-metals are usually assigned as follows:
- Hydrogen: +1 when combined with nonmetals, −1 when combined with metals
- Oxygen: −2 in most compounds, sometimes −1 (so-called peroxides, O22−), very rarely [latex]-\frac{1}{2}[/latex] (so-called superoxides, O2−), positive values when combined with F (values vary)
- Halogens: −1 for F always, −1 for other halogens except when combined with oxygen or other halogens (positive oxidation numbers in these cases, varying values)
- The sum of oxidation numbers for all atoms in a molecule or polyatomic ion equals the charge on the molecule or ion.
Example 1
Follow the guidelines in this section of the text to assign oxidation numbers to all the elements in the following species:
a) H2S
b) SO32−
Solution
a) According to guideline 1, the oxidation number for H is +1.
Using this oxidation number and the compound’s formula, guideline 4 may then be used to calculate the oxidation number for sulfur:
b) Guideline 3 suggests the oxidation number for oxygen is −2.
Using this oxidation number and the ion’s formula, guideline 4 may then be used to calculate the oxidation number for sulfur:
Using the oxidation number concept, an all-inclusive definition of redox reaction has been established. Oxidation-reduction (redox) reactions are those in which one or more elements involved undergo a change in oxidation number.
Returning to the reactions used to introduce this topic, they may now both be identified as redox processes. In the reaction between sodium and chlorine to yield sodium chloride, sodium is oxidized (its oxidation number increases from 0 in Na to +1 in NaCl) and chlorine is reduced (its oxidation number decreases from 0 in Cl2 to −1 in NaCl). In the reaction between molecular hydrogen and chlorine, hydrogen is oxidized (its oxidation number increases from 0 in H2 to +1 in HCl) and chlorine is reduced (its oxidation number decreases from 0 in Cl2 to −1 in HCl).
Some Important Types of Redox Reactions
1 – A composition reaction (sometimes also called a combination reaction or a synthesis reaction) produces a single substance from multiple reactants. A single substance as a product is the key characteristic of the composition reaction. There may be a coefficient other than one for the substance, but if the reaction has only a single substance as a product, it can be called a composition reaction. In the reaction
2 H2(g) + O2(g) [latex]\longrightarrow[/latex] 2 H2O(ℓ)
water is produced from hydrogen and oxygen. Although there are two molecules of water being produced, there is only one substance—water—as a product. So this is a composition reaction.
2 – A decomposition reaction starts from a single substance and produces more than one substance; that is, it decomposes. One substance as a reactant and more than one substance as the products is the key characteristic of a decomposition reaction. For example, in the decomposition of sodium hydrogen carbonate (also known as sodium bicarbonate),
2 NaHCO3(s) [latex]\longrightarrow[/latex] Na2CO3(s) + CO2(g) + H2O(ℓ)
sodium carbonate, carbon dioxide, and water are produced from the single substance sodium hydrogen carbonate.
Composition and decomposition reactions are difficult to predict; however, they should be easy to recognize.
Example 3
Identify each equation as a composition reaction, a decomposition reaction, or neither.
a) Fe2O3 + 3 SO3[latex]\longrightarrow[/latex] Fe2(SO4)3
b) NaCl + AgNO3 [latex]\longrightarrow[/latex] AgCl + NaNO3
c) (NH4)2Cr2O7 [latex]\longrightarrow[/latex] Cr2O3 + 4 H2O + N2
Solution
a) In this equation, two substances combine to make a single substance. This is a composition reaction.
b) Two different substances react to make two new substances. This does not fit the definition of either a composition reaction or a decomposition reaction, so it is neither. In fact, you may recognize this as a double-replacement reaction.
c) A single substance reacts to make multiple substances. This is a decomposition reaction.
Test Yourself
Identify the equation as a composition reaction, a decomposition reaction, or neither.
C3H8 [latex]\longrightarrow[/latex] C3H4 + 2 H2
Answer
decomposition
3 – Combustion reactions in which the reductant, also called a fuel, and oxidant, molecular oxygen, react vigorously and produce significant amounts of heat, and often light, in the form of a flame. Combustion reactions produce oxides of all other elements as products; any nitrogen in the reactant is converted to elemental nitrogen, N2. Many reactants, called fuels, contain mostly carbon and hydrogen atoms, reacting with oxygen to produce CO2 and H2O. For example, the balanced chemical equation for the combustion of methane, CH4, is as follows:
CH4 + 2 O2 [latex]\longrightarrow[/latex] CO2 + 2 H2O
Kerosene can be approximated with the formula C12H26, and its combustion equation is
2 C12H26 + 37 O2 [latex]\longrightarrow[/latex] 24 CO2 + 26 H2O
Sometimes fuels contain oxygen atoms, which must be counted when balancing the chemical equation. One common fuel is ethanol, C2H5OH, whose combustion equation is
C2H5OH + 3 O2 [latex]\longrightarrow[/latex] 2 CO2 + 3 H2O
If nitrogen is present in the original fuel, it is converted to N2, not to a nitrogen-oxygen compound. Thus, for the combustion of the fuel dinitroethylene, whose formula is C2H2N2O4, we have
2 C2H2N2O4 + O2 [latex]\longrightarrow[/latex] 4 CO2 + 2 H2O + 2 N2
Example 4
Complete and balance each combustion equation.
a) the combustion of propane, C3H8
b) the combustion of ammonia, NH3

Solution
a) The products of the reaction are CO2 and H2O, so our unbalanced equation is
C3H8 + O2 [latex]\longrightarrow[/latex] CO2 + H2O
Balancing (and you may have to go back and forth a few times to balance this), we get
C3H8 + 5 O2 [latex]\longrightarrow[/latex] 3 CO2 + 4 H2O
b) The nitrogen atoms in ammonia will react to make N2, while the hydrogen atoms will react with O2 to make H2O:
NH3 + O2 [latex]\longrightarrow[/latex] N2 + H2O
To balance this equation without fractions (which is the convention), we get
4 NH3 + 3 O2 [latex]\longrightarrow[/latex] 2 N2 + 6 H2O
Test Yourself
Complete and balance the combustion equation for cyclopropanol, C3H6O.
Answer
C3H6O + 4 O2 [latex]\longrightarrow[/latex] 3 CO2 + 3 H2O

Watch a brief video showing the test firing of a small-scale, prototype, hybrid rocket engine planned for use in a Space Launch System at NASA. The first engines firing at 3 s (green flame) use a liquid fuel/oxidant mixture, and the second, more powerful engines firing at 4 s (yellow flame) use a solid mixture.
4 – Single-displacement (replacement) reactions are redox reactions in which an ion in solution is displaced (or replaced) via the oxidation of a metallic element. One common example of this type of reaction is the acid oxidation of certain metals:
Metallic elements may also be oxidized by solutions of other metal salts; for example:
This reaction may be observed by placing copper wire in a solution containing a dissolved silver salt. Silver ions in solution are reduced to elemental silver at the surface of the copper wire, and the resulting Cu2+ ions dissolve in the solution to yield a characteristic blue color (Figure 2).
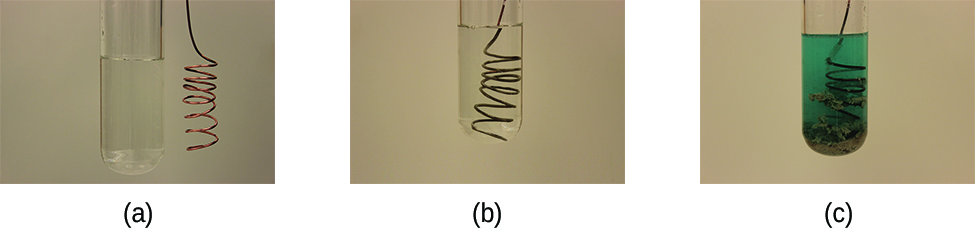
Example 5
Identify which equations represent redox reactions, providing a name for the reaction if appropriate. For those reactions identified as redox, name the oxidant and reductant.
a) [latex]\text{ZnCO}_3(s) \longrightarrow \text{ZnO}(s) + \text{CO}_2(g)[/latex]
b) [latex]2\text{Ga}(l) + 3\text{Br}_2(l) \longrightarrow 2\text{GaBr}_3(s)[/latex]
c) [latex]\text{C}_2 \text{H}_4(g) + 3\text{O}_2(g) \longrightarrow 2\text{CO}_2(g) + 2\text{H}_2 \text{O}(l)[/latex]
Solution
Redox reactions are identified per definition if one or more elements undergo a change in oxidation number.
a) This is not a redox reaction, since oxidation numbers remain unchanged for all elements.
b) This is a redox reaction. Gallium is oxidized, its oxidation number increasing from 0 in Ga(l) to +3 in GaBr3(s). Bromine is reduced, its oxidation number decreasing from 0 in Br2(l) to −1 in GaBr3(s).
c) This is a redox reaction (combustion). Carbon is oxidized, its oxidation number increasing from −2 in C2H4(g) to +4 in CO2(g). The reducing agent (fuel) is C2H4(g). Oxygen is reduced, its oxidation number decreasing from 0 in O2(g) to −2 in H2O(l). The oxidizing agent is O2(g).
Key Concepts and Summary
Chemical reactions are classified according to similar patterns of behavior. Redox reactions involve a change in oxidation number for one or more reactant elements. Some important reaction types are redox reactions, including composition, decomposition, combustion and single displacement reactions.
Review-Reflect, Extend
1. Is the reaction
2 K(s) + Br2(ℓ) [latex]\longrightarrow[/latex] 2 KBr(s)
an oxidation-reduction reaction? Explain your answer.
2. In the reaction
2 Ca(s) + O2(g) [latex]\longrightarrow[/latex] 2 CaO(s)
indicate what has lost electrons and what has gained electrons.
3. In the reaction
2 Li(s) + O2(g) [latex]\longrightarrow[/latex] Li2O2(s)
indicate what has been oxidized and what has been reduced.
4. Assign oxidation numbers to each atom in each substance.
a) CO b) CO2
c) NiCl2 d) NiCl3
5. Indicate what type, or types, of reaction each of the following represents:
a) [latex]\text{Ca}(s) + \text{Br}_2(l) \longrightarrow \text{CaBr}_2(s)[/latex ]
b) [latex ]\text{Ca(OH)}_2 (aq) + 2\text{HBr}(aq) \longrightarrow \text{CaBr}_2(aq) + 2\text{H}_2 \text{O}(l)[/latex ]
c) [latex ] \text{C}_6 \text{H}_{12}(l) + 9\text{O}_2(g) \longrightarrow 6\text{CO}_2(g) + 6\text{H}_2 \text{O}(g)[/latex ]
6. Silver can be separated from gold because silver dissolves in nitric acid while gold does not. Is the dissolution of silver in nitric acid an acid-base reaction or an oxidation-reduction reaction? Explain your answer. 7. Which is a composition reaction and which is not?
a) NaCl + AgNO3 [latex ]\longrightarrow[/latex] AgCl + NaNO3
b) CaO + CO2 [latex]\longrightarrow[/latex] CaCO3
8. Which is a decomposition reaction and which is not?
a) HCl + NaOH [latex]\longrightarrow[/latex] NaCl + H2O
b) CaCO3 [latex]\longrightarrow[/latex] CaO + CO2
9. Which is a combustion reaction and which is not?
a) C6H12O6 + 6 O2 [latex]\longrightarrow[/latex] 6 CO2 + 6 H2O
b) 2 Fe2S3 + 9 O2 [latex]\longrightarrow[/latex] 2 Fe2O3 + 6 SO2
10. Complete and balance each combustion equation.
a) C4H9OH + O2 [latex]\longrightarrow[/latex] ?
b) CH3NO2 + O2 [latex]\longrightarrow[/latex] ?
Answers
1. Yes; both K and Br are changing oxidation numbers.
2. Ca has lost electrons, and O has gained electrons.
3. Li has been oxidized, and O has been reduced.
4. a) C: +2; O: −2
b) C: +4; O: −2
c) Ni: +2; Cl: −1
d) Ni: +3; Cl: −1
5. a) oxidation-reduction (addition); b) acid-base (neutralization); c) oxidation-reduction (combustion)
6. It is an oxidation-reduction reaction because the oxidation state of the silver changes during the reaction.
7. a) not composition b) composition
8. a) not decomposition b) decomposition
9. a) combustion b) combustion
10. a) C4H9OH + 6 O2 [latex]\longrightarrow[/latex] 4 CO2 + 5 H2O
Glossary
combustion reaction: vigorous redox reaction producing significant amounts of energy in the form of heat and, sometimes, light
half-reaction: an equation that shows whether each reactant loses or gains electrons in a reaction.
oxidation: process in which an element’s oxidation number is increased by loss of electrons
oxidation-reduction reaction: (also, redox reaction) reaction involving a change in oxidation number for one or more reactant elements
oxidation number: (also, oxidation state) the charge each atom of an element would have in a compound if the compound were ionic
oxidizing agent: (also, oxidant) substance that brings about the oxidation of another substance, and in the process becomes reduced
reduction: process in which an element’s oxidation number is decreased by gain of electrons
reducing agent: (also, reductant) substance that brings about the reduction of another substance, and in the process becomes oxidized
single-displacement reaction: (also, replacement) redox reaction involving the oxidation of an elemental substance by an ionic species

