9 2.4 Chemical Formulas
Learning Objectives
By the end of this section, you will be able to:
- Symbolize the composition of molecules using molecular formulas
- Represent the bonding arrangement of atoms within molecules using structural formulas
There is a rich and complex symbolic language used in chemistry. Because atoms and molecules are too small to see, this language is used extensively to share and discuss chemical ideas. All of the various representations used are models: approximations of the real atoms and molecules under discussion. Each type of representation has its own level of detail, its own accuracies and inaccuracies. Like models we use in other parts of our lives, these representations are helpful to us even if they aren’t exactly like the real thing.
We have already been introduced to symbols for elements, atoms, ions and isotopes. Now we will investigate some of the ways molecules and compounds are described.
A molecular formula is a representation of a molecule that uses chemical symbols to indicate the types of atoms followed by subscripts to show the number of atoms of each type in the molecule. (A subscript is used only when more than one atom of a given type is present.) Molecular formulas are also used as abbreviations for the names of compounds.
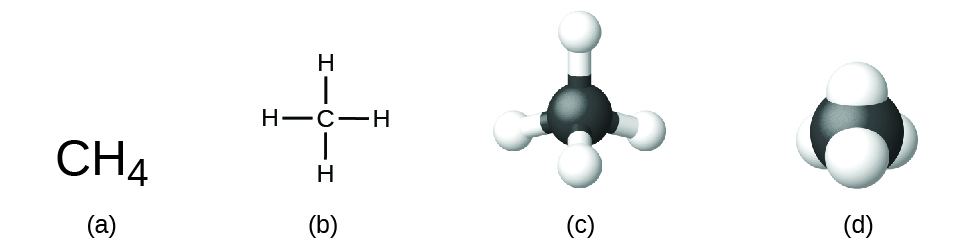
Various types of structural formula for a compound give the same information as the molecular formula, but also shows how the atoms are connected in the molecule. A structural formula for methane contains symbols for one C atom and four H atoms, indicating the number of atoms in the molecule (Figure 1). The lines represent attractive forces called chemical bonds that hold the atoms together. For now, we do not need to investigate the nature of these bonds. A ball-and-stick model shows the geometric arrangement of the atoms with atomic sizes not to scale, and a space-filling model shows the relative sizes of the atoms.
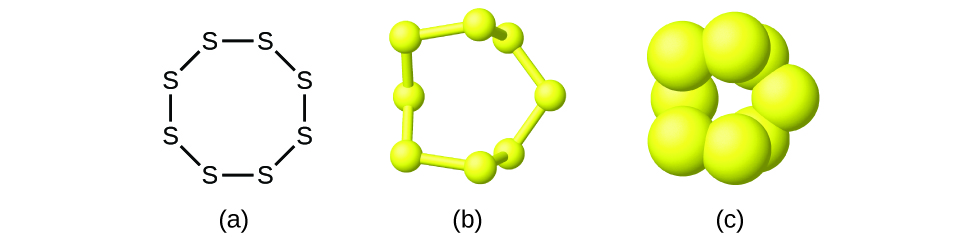
Although many elements consist of discrete, individual atoms, some exist as molecules made up of two or more atoms of the element chemically bonded together. For example, most samples of the elements hydrogen, oxygen, and nitrogen are composed of molecules that contain two atoms each (called diatomic molecules) and thus have the molecular formulas H2, O2, and N2, respectively. Other elements commonly found as diatomic molecules are fluorine (F2), chlorine (Cl2), bromine (Br2), and iodine (I2). The most common form of the element sulfur is composed of molecules that consist of eight atoms of sulfur; its molecular formula is S8 (Figure 2).
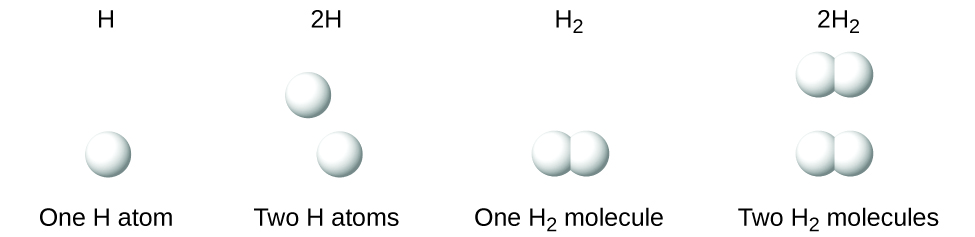
Note that in a molecule with more than one atom of a particular element, the number of atoms of that element is subscripted. H2 and 2H represent distinctly different species. H2 is a molecular formula; it represents a diatomic molecule of hydrogen, consisting of two atoms of the element that are chemically bonded together. The expression 2H, on the other hand, indicates two separate hydrogen atoms that are not combined as a unit. The expression 2H2 represents two molecules of diatomic hydrogen (Figure 3).
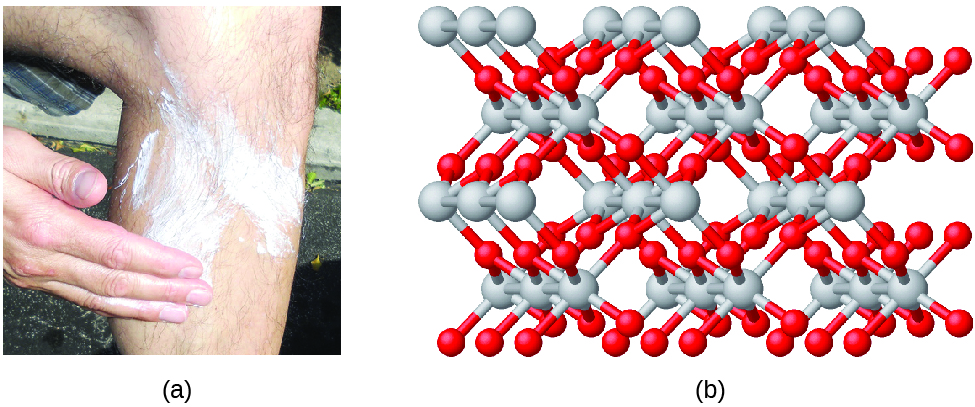
Compounds are formed when two or more elements chemically combine, resulting in the formation of bonds. For example, hydrogen and oxygen can react to form water, and sodium and chlorine can react to form table salt. We sometimes describe the composition of these compounds with an empirical formula, which indicates the types of atoms present and the simplest whole-number ratio of the number of atoms (or ions) in the compound. For example, titanium dioxide (used as pigment in white paint and in the thick, white, blocking type of sunscreen) has an empirical formula of TiO2. This identifies the elements titanium (Ti) and oxygen (O) as the constituents of titanium dioxide, and indicates the presence of twice as many atoms of the element oxygen as atoms of the element titanium (Figure 4).
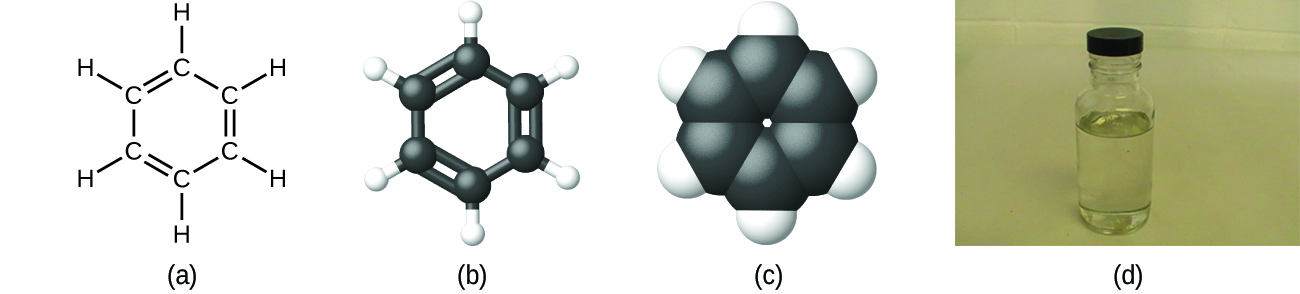
As discussed previously, we can describe a compound with a molecular formula, in which the subscripts indicate the actual numbers of atoms of each element in a molecule of the compound. For example, an experimental determination of the molecular mass reveals that a molecule of benzene contains six carbon atoms and six hydrogen atoms, so while the empirical formula for this substance would be CH, the molecular formula for benzene is C6H6 (Figure 5).
Example 1
Molecules of glucose (blood sugar) contain 6 carbon atoms, 12 hydrogen atoms, and 6 oxygen atoms. What are the molecular formula of glucose?
Solution
The molecular formula is C6H12O6 because one molecule actually contains 6 C, 12 H, and 6 O atoms.

What is it that chemists do? According to Lee Cronin, chemists make very complicated molecules by “chopping up” small molecules and “reverse engineering” them. He wonders if we could “make a really cool universal chemistry set” by what he calls “app-ing” chemistry. Could we “app” chemistry?
In a 2012 TED talk, Lee describes one fascinating possibility: combining a collection of chemical “inks” with a 3D printer capable of fabricating a reaction apparatus (tiny test tubes, beakers, and the like) to fashion a “universal toolkit of chemistry.” This toolkit could be used to create custom-tailored drugs to fight a new superbug or to “print” medicine personally configured to your genetic makeup, environment, and health situation. Says Cronin, “What Apple did for music, I’d like to do for the discovery and distribution of prescription drugs.”[1] View his full talk at the TED website.
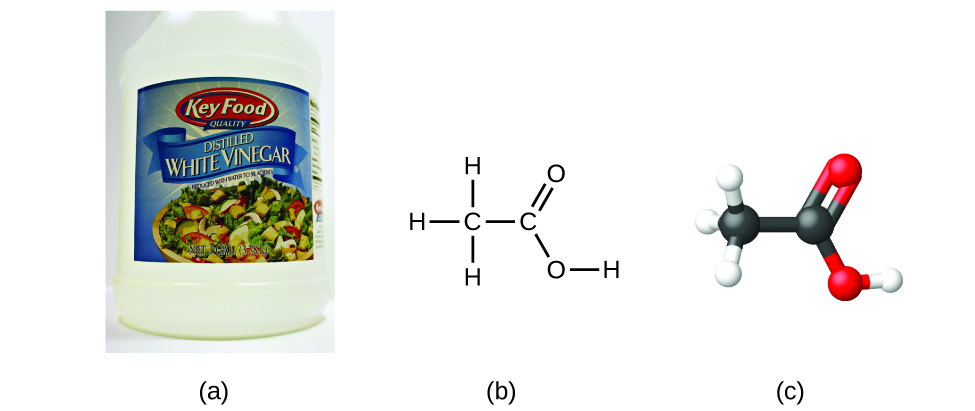
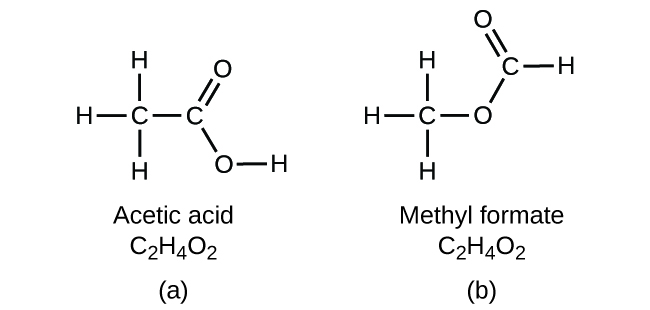
It is important to be aware that it may be possible for the same atoms to be arranged in different ways: Compounds with the same molecular formula may have different atom-to-atom bonding and therefore different structures. For example, could there be another compound with the same formula as acetic acid, C2H4O2? And if so, what would be the structure of its molecules?
Another compound with the formula C2H4O2 does exist.Two C atoms, four H atoms, and two O atoms can also be arranged to form a methyl formate, which is used in manufacturing, as an insecticide, and for quick-drying finishes. Methyl formate molecules have one of the oxygen atoms between the two carbon atoms, differing from the arrangement in acetic acid molecules. Acetic acid and methyl formate are examples of isomers—compounds with the same chemical formula but different molecular structures (Figure 7). Note that this small difference in the arrangement of the atoms has a major effect on their respective chemical properties. You would certainly not want to use a solution of methyl formate as a substitute for a solution of acetic acid (vinegar) when you make salad dressing.
Key Concepts and Summary
The symbolic representations used in chemistry are numerous and varied. All of them are models of real molecules, with different levels of accuracy and detail. A molecular formula uses chemical symbols and subscripts to indicate the exact numbers of different atoms in a molecule or compound. An empirical formula gives the simplest, whole-number ratio of atoms in a compound. A structural formula indicates the bonding arrangement of the atoms in the molecule. Ball-and-stick and space-filling models show the geometric arrangement of atoms in a molecule. Isomers are compounds with the same molecular formula but different arrangements of atoms.
Review-Reflect, Extend
Review-Reflect
1. Explain why the symbol for an atom of the element oxygen and the formula for a molecule of oxygen differ.
2. Write the molecular formulas of the following compounds:
a)
![]()
b)
![]()
c)
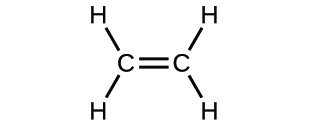
d)
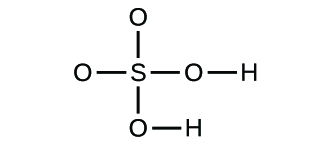
3. How many different elements, and how many atoms of each type of element, are present in these compounds?
a) caffeine, C8H10N4O2
b) fructose, C12H22O11
c) hydrogen peroxide, H2O2
d) glucose, C6H12O6
e) ascorbic acid (vitamin C), C6H8O6
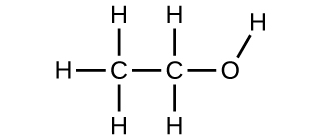
4. Use the Build a Molecule simulation to build a molecule with two carbons, six hydrogens, and one oxygen.
a) Draw the structural formula of this molecule and state its name.
b) Can you arrange these atoms to make a different molecule? If so, draw its structural formula and state its name.
c) How are the molecules drawn in (a) and (b) the same? How do they differ? What are they called (the type of relationship between these molecules, not their names).
Answers
1. The symbol for the element oxygen, O, represents both the element and one atom of oxygen. A molecule of oxygen, O2, contains two oxygen atoms; the subscript 2 in the formula must be used to distinguish the diatomic molecule from two single oxygen atoms.
2. a) molecular CO2, empirical CO2 b) molecular C2H2, empirical CH
c) molecular C2H4, empirical CH2 d) molecular H2SO4, empirical H2SO4
3.
a) carbon: 4 hydrogen: 5 nitrogen 2 oxygen 1
b) carbon: 12 hydrogen: 22 oxygen: 11
c) hydrogen: 2 oxygen: 2
d) carbon: 6 hydrogen: 12 oxygen: 6
e) carbon: 6 hydrogen: 8 oxygen: 6
4. a) ethanol
b) methoxymethane, more commonly known as dimethyl ether
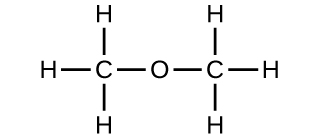
c) These molecules have the same chemical composition (types and number of atoms) but different chemical structures. They are structural isomers.
Glossary
empirical formula: formula showing the composition of a compound given as the simplest whole-number ratio of atoms
isomers: compounds with the same chemical formula but different structures
molecular formula: formula indicating the composition of a molecule of a compound and giving the actual number of atoms of each element in a molecule of the compound.
spatial isomers: compounds in which the relative orientations of the atoms in space differ
structural formula: shows the atoms in a molecule and how they are connected
structural isomer: one of two substances that have the same molecular formula but different physical and chemical properties because their atoms are bonded differently
- Lee Cronin, “Print Your Own Medicine,” Talk presented at TED Global 2012, Edinburgh, Scotland, June 2012. ↵

