4 1.3 Physical and Chemical Properties
Learning Objectives
By the end of this section, you will be able to:
- Identify properties of and changes in matter as physical or chemical
- Identify properties of matter as extensive or intensive
Recall that chemistry is the study of matter, its properties, the changes that matter undergoes and the energy associated with these changes. Note that energy itself is not matter, but comes in a variety of forms such as heat, light, sound, or in the kinetic energy of moving matter.
When matter undergoes change, the process is often accompanied by a change in energy. Heat can be released during a change, which is called exothermic. Reactions such as those that involve the combustion of fuels are familiar examples of exothermic reactions. When heat is needs to be supplied to make a process occur the change is called endothermic. These processes are less familiar to us, though some occur commonly. The cooling that occurs as sweat evaporates from our skin is an endothermic change of state from liquid water in the sweat to gas.
An important distinction is that heat is energy that flows due to a temperature difference, while temperature is an indication or measure of the average kinetic energy of the molecules in a substance. The faster they move, the “hotter” it is. So as heat flows into a substance its temperature will rise.
The characteristics that enable us to distinguish one substance from another are called properties. A physical property is a characteristic of matter that is not associated with a change in its chemical composition. Familiar examples of physical properties include density, color, hardness, melting and boiling points. We can observe some physical properties, such as density and color, without changing the physical state of the matter observed. Other physical properties, such as the melting temperature of iron or the freezing temperature of water, can only be observed as matter undergoes a physical change. A physical change is a change in the state (Figure 1) or properties of matter without any accompanying change in its chemical composition (the identities of the substances contained in the matter), such as the melting and freezing described above.
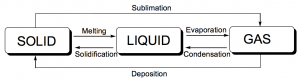
We observe a physical change when wax melts, when sugar dissolves in coffee, and when steam condenses into liquid water (Figure 2). Other examples of physical changes include magnetizing and demagnetizing metals (as is done with common antitheft security tags) and grinding solids into powders (which can sometimes yield noticeable changes in color). In each of these examples, there is a change in the physical state, form, or properties of the substance, but no change in its chemical composition.

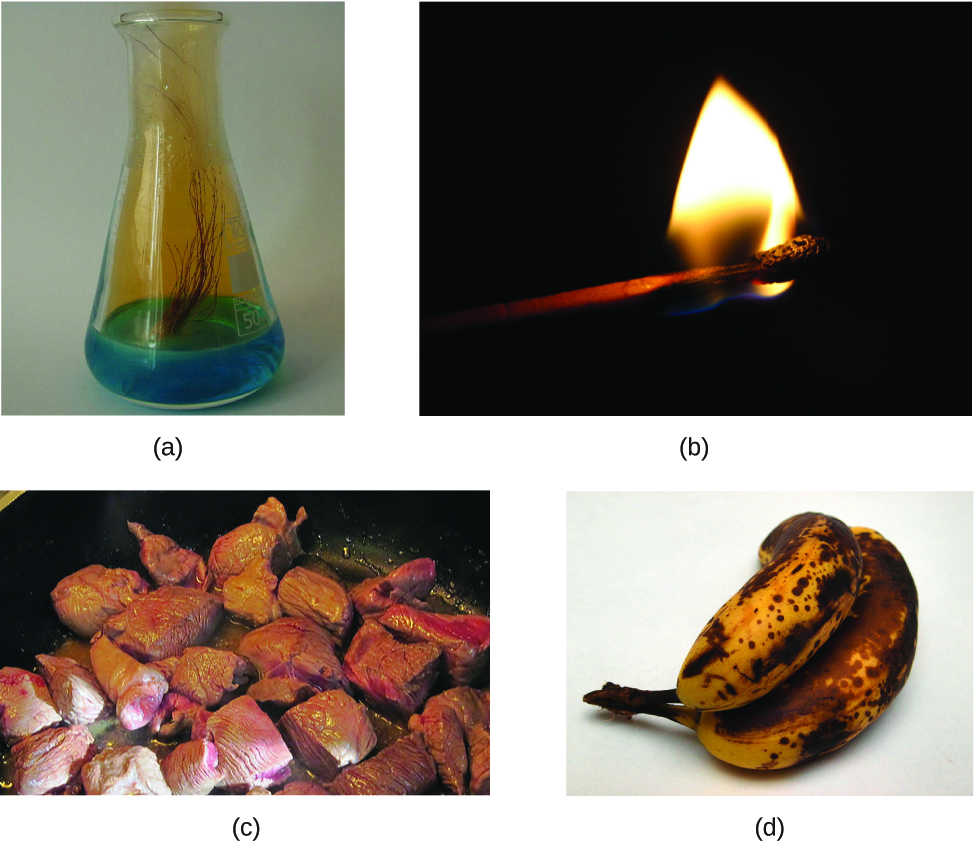
The change of one type of matter into another type (or the inability to change) is a chemical property. Examples of chemical properties include flammability, acidity, reactivity (many types), and heat of combustion. Iron, for example, combines with oxygen in the presence of water to form rust; chromium does not oxidize (Figure 3). Nitroglycerin explodes when impacted; neon poses almost no hazard because it is very unreactive.

To identify a chemical property, we look for a chemical change. A chemical change always produces one or more types of matter that differ from the matter present before the change. The formation of rust is a chemical change because rust is a different kind of matter than the iron, oxygen, and water present before the rust formed. The explosion of nitroglycerin is a chemical change because the gases produced are very different kinds of matter from the original substance. Other examples of chemical changes include reactions that are performed in a lab (such as copper reacting with nitric acid), all forms of combustion (burning), and food being cooked, digested, or rotting (Figure 4).
Example 1
Classify each of the following as either a physical property, or a chemical property:
a) The boiling point of water is 100oC
b) Oxygen is a gas under normal room conditions
c) Sugar ferments to form alcohol
Solution
a) Although this property describes a change, this change does not involve a change in substance. H2O remains H2O despite what state it is in. Thus, this is a physical property.
b) This is an inherent property, and is therefore a physical property.
c) This property involves a change in substance, from sugar to alcohol. This is a chemical property.
Properties of matter fall into one of two categories. If the property depends on the amount of matter present, it is an extensive property. The mass and volume of a substance are examples of extensive properties; for instance, a gallon of milk has a larger mass and volume than a cup of milk. If the property of a sample of matter does not depend on the amount of matter present, it is an intensive property. Temperature is an example of an intensive property. If the gallon and cup of milk are each at 20 °C (room temperature), when they are combined, the temperature remains at 20 °C.
Example 2
Classify each of the following as either a physical change, or a chemical change:
a) Steam condensing on a shower mirror
b) Iron forming rust
c) An antacid tablet fizzes when it comes in contact with stomach acid
Solution
a) The steam is water vapor, and when it condenses, it forms liquid water on the mirror.
This is a physical change.
b) Iron reacts with the oxygen in air, forming an iron oxide, which is rust.
This is a chemical change.
c) The fizzing in the water is the release of carbon dioxide gas when it comes in contact with acid. This is a chemical change.
Example 3
Describe each process as a physical change or a chemical change.
a) Water in the air turns into snow.
b) A person’s hair is cut.
c) Bread dough becomes fresh bread in an oven.
Solution
a) Because the water is going from a gas phase to a solid phase, this is a physical change.
b) Your long hair is being shortened. This is a physical change.
c) Because of the oven’s temperature, chemical changes are occurring in the bread dough to make fresh bread. These are chemical changes. (In fact, a lot of cooking involves chemical changes.)
Test Yourself
Identify each process as a physical change or a chemical change.
a) A fire is raging in a fireplace.
b) Water is warmed to make a cup of coffee.
Answers
a) chemical change b) physical change
Hazard Diamond
You may have seen the symbol shown in Figure 5 on containers of chemicals in a laboratory or workplace. Sometimes called a “fire diamond” or “hazard diamond,” this chemical hazard diamond provides valuable information that briefly summarizes the various dangers of which to be aware when working with a particular substance.
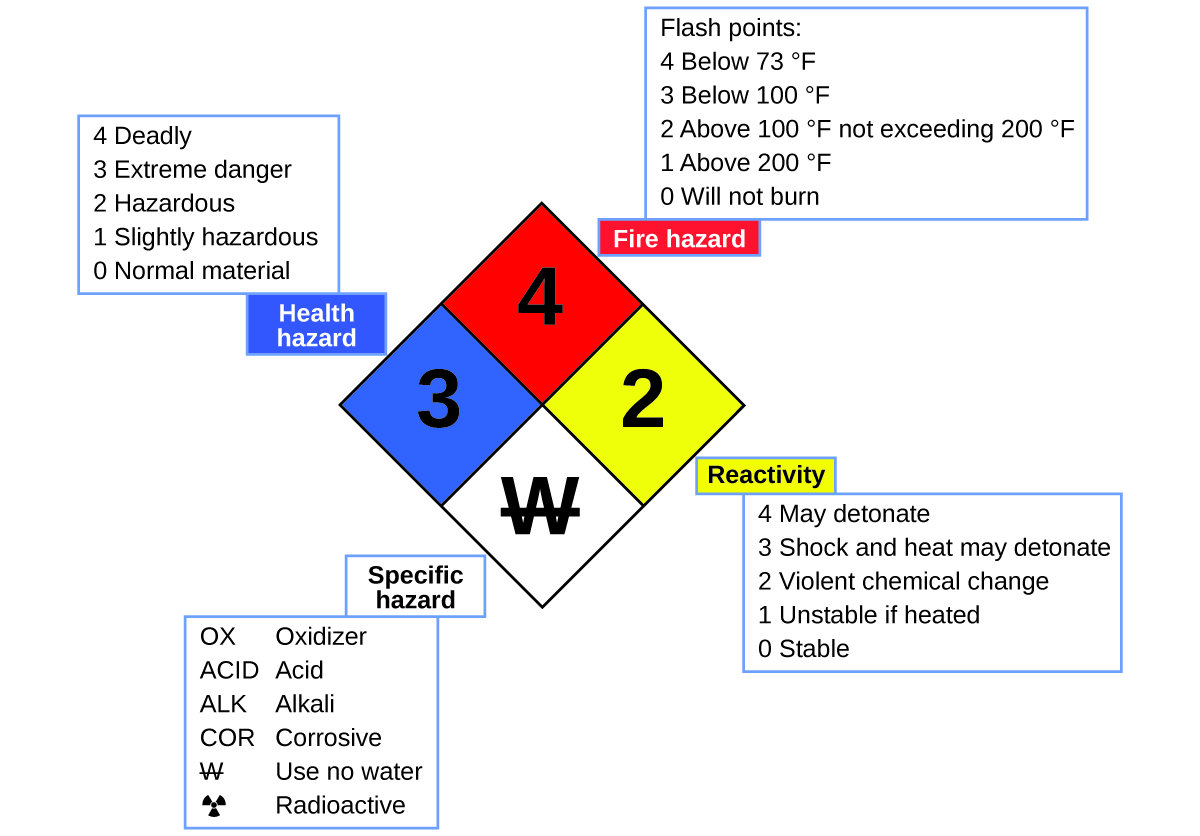
The National Fire Protection Agency (NFPA) 704 Hazard Identification System was developed by NFPA to provide safety information about certain substances. The system details flammability, reactivity, health, and other hazards. Within the overall diamond symbol, the top (red) diamond specifies the level of fire hazard (temperature range for flash point). The blue (left) diamond indicates the level of health hazard. The yellow (right) diamond describes reactivity hazards, such as how readily the substance will undergo detonation or a violent chemical change. The white (bottom) diamond points out special hazards, such as if it is an oxidizer (which allows the substance to burn in the absence of air/oxygen), undergoes an unusual or dangerous reaction with water, is corrosive, acidic, alkaline, a biological hazard, radioactive, and so on. Each hazard is rated on a scale from 0 to 4, with 0 being no hazard and 4 being extremely hazardous.
Decomposition of Water / Production of Hydrogen
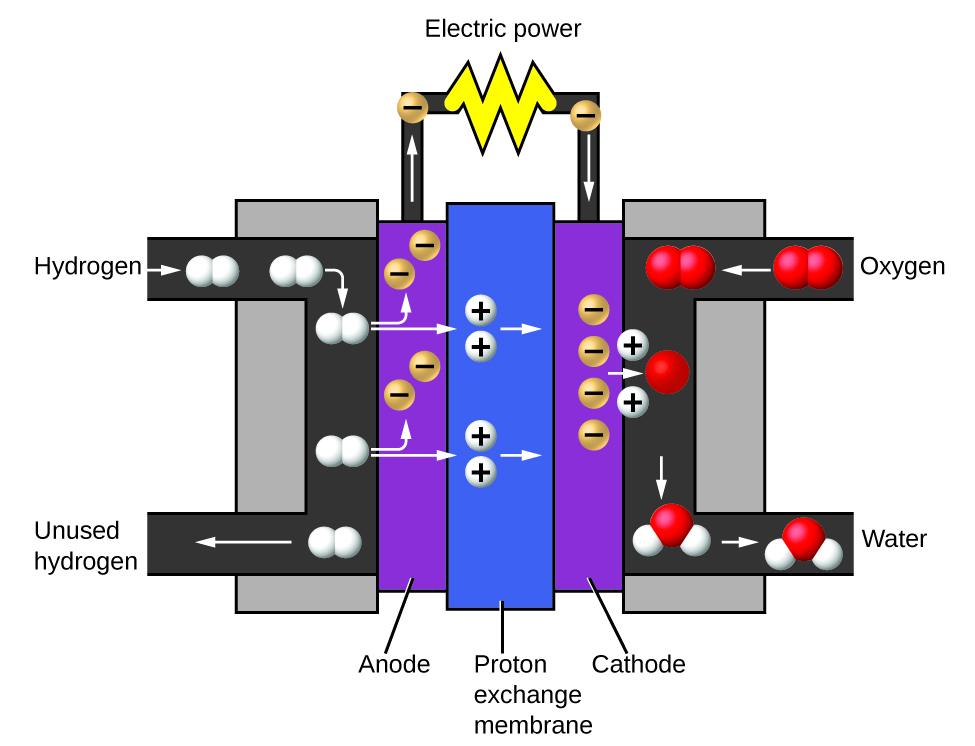
Water consists of the elements hydrogen and oxygen combined in a 2 to 1 ratio. Water can undergo a chemical change involving the water molecules being broken down into hydrogen and oxygen gases by the addition of energy. One way to do this is with a battery or power supply, as shown in (Figure 6).
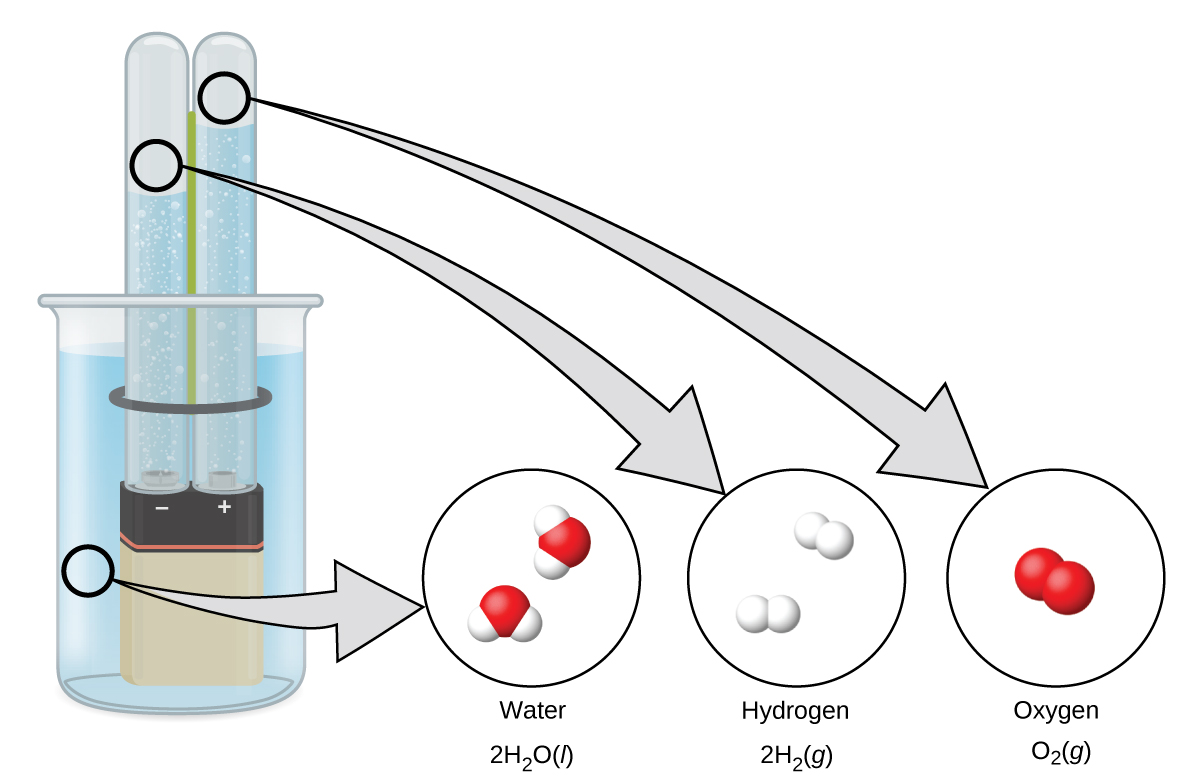
The breakdown of water involves a rearrangement of the atoms in water molecules into different molecules, each composed of two hydrogen atoms and two oxygen atoms, respectively. Two water molecules form one oxygen molecule and two hydrogen molecules. The representation for what occurs, [latex]2\text{H}_2\text{O}(l) \rightarrow 2\text{H}_2(g) + \text{O}_2(g)[/latex] , will be explored in more depth in later chapters.
The two gases produced have distinctly different properties. Oxygen is not flammable but is required for combustion of a fuel, and hydrogen is highly flammable and a potent energy source. How might this knowledge be applied in our world? One application involves research into more fuel-efficient transportation. Fuel-cell vehicles (FCV) run on hydrogen instead of gasoline (Figure 7). They are more efficient than vehicles with internal combustion engines, are nonpolluting, and reduce greenhouse gas emissions, making us less dependent on fossil fuels. FCVs are not yet economically viable, however, and current hydrogen production depends on natural gas. If we can develop a process to economically decompose water, or produce hydrogen in another environmentally sound way, FCVs may be the way of the future.
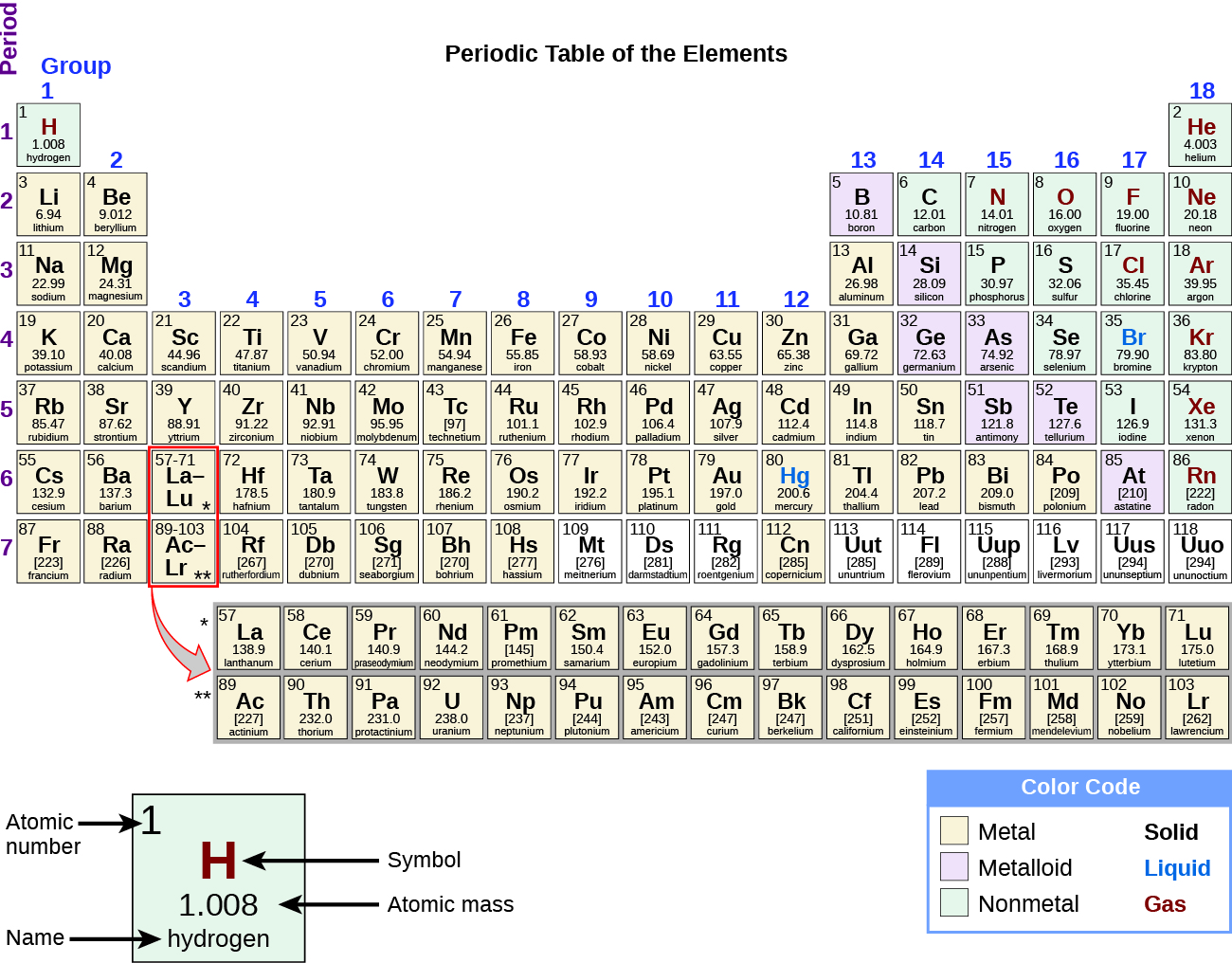
While many elements differ dramatically in their chemical and physical properties, some elements have similar properties. We can identify sets of elements that exhibit common behaviors. For example, many elements conduct heat and electricity well, whereas others are poor conductors. These properties can be used to sort the elements into three classes: metals (elements that conduct well), nonmetals (elements that conduct poorly), and metalloids (elements that have properties of both metals and nonmetals).
The periodic table is a table of elements that places elements with similar properties close together (Figure 6). You will learn more about the periodic table as you continue your study of chemistry.
Key Concepts and Summary
All substances have distinct physical and chemical properties, and may undergo physical or chemical changes. Physical properties, such as hardness and boiling point, and physical changes, such as melting or freezing, do not involve a change in the composition of matter. Chemical properties, such flammability and acidity, and chemical changes, such as rusting, involve production of matter that differs from that present beforehand.
Measurable properties fall into one of two categories. Extensive properties depend on the amount of matter present, for example, the mass of gold. Intensive properties do not depend on the amount of matter present, for example, the density of gold.
Review-Reflect, Extend
Review-Reflect
1. Classify each of the following changes as physical or chemical:
a) condensation of steam
b) burning of gasoline
c) souring of milk
d) dissolving of sugar in water
e) melting of gold
2. The volume of a sample of oxygen gas changed from 10 mL to 11 mL as the temperature changed. Is this a chemical or physical change?
3. Explain the difference between extensive properties and intensive properties.
4. The density (d) of a substance is an intensive property that is defined as the ratio of its mass (m) to its volume (V).
Considering that mass and volume are both extensive properties, explain why their ratio, density, is intensive.
5. Does each statement represent a physical property or a chemical property?
a) Sulfur is yellow.
b) Steel wool burns when ignited by a flame.
c) A gallon of milk weighs over eight pounds.
6. Does each statement represent a physical property or a chemical property?
a) A pile of leaves slowly rots in the backyard.
b) In the presence of oxygen, hydrogen can interact to make water.
c) Gold can be stretched into very thin wires.
7. Does each statement represent a physical change or a chemical change?
a) Water boils and becomes steam.
b) Food is converted into usable form by the digestive system.
c) The alcohol in many thermometers freezes at about −40 degrees Fahrenheit.
8. Does each statement represent a physical change or a chemical change?
a) Graphite, a form of elemental carbon, can be turned into diamond, another form of carbon, at very high temperatures and pressures.
b) The elements sodium and chlorine come together to make a new substance called sodium chloride.
Extend
9. Is heat an extensive or intensive property? Is temperature?
Consider: A drop of hot cooking oil spattered on your arm causes brief, minor discomfort, whereas a pot of hot oil yields severe burns.
Answers
1. a) physical; b) chemical; c) chemical; d) dissolving is considered physical since the restoration of the original substance can occur through physical separation of the components of the mixture. In this case imagine evaporating off the water, which restores the original sugar with all its properties intact. e) physical
2. physical
3. The value of an extensive property depends upon the amount of matter being considered, whereas the value of an intensive property is the same regardless of the amount of matter being considered.
4. Being extensive properties, both mass and volume are directly proportional to the amount of substance under study. Dividing one extensive property by another will in effect “cancel” this dependence on amount, yielding a ratio that is independent of amount (an intensive property).
5. a) physical property b) chemical property c) physical property
6. a) chemical property b) chemical property c) physical property
7. a) physical change b) chemical change c) physical change
8. a) physical change b) chemical change
Glossary
chemical change: change producing a different kind of matter from the original kind of matter
chemical property: behavior that is related to the change of one kind of matter into another kind of matter
endothermic: if heat is needs to be supplied, for a change to occur
energy: the ability to do “work”— that is, for a force to act on something and push some distance
exothermic: if heat is released during a change
extensive property: property of a substance that depends on the amount of the substance
intensive property: property of a substance that is independent of the amount of the substance
physical change: change in the state or properties of matter that does not involve a change in its chemical composition
physical property: characteristic of matter that is not associated with any change in its chemical composition

