10.3 Acid Rain
Section Goals:
- Connect air pollution with acid rain.
- Understand ecological and economic effects of acid rain.
Acid Rain
Acid–base reactions can have a strong environmental impact. For example, a dramatic increase in the acidity of rain and snow over the past 150 years is dissolving marble and limestone surfaces, accelerating the corrosion of metal objects, and decreasing the pH of natural waters. This environmental problem is called acid rain and has significant consequences for all living organisms. Acid rain is a term referring to a mixture of wet and dry deposition (deposited material) from the atmosphere containing higher than normal amounts of nitric and sulfuric acids. Recall that pH is a scale based on the quantity of H+ ions present (Section 2.1). The lower a substance’s pH, the more acidic it is. Pure water has a pH of 7.0.
To understand acid rain requires an understanding of acid–base reactions in aqueous solution. The term acid rain is actually somewhat misleading because even pure rainwater collected in areas remote from civilization is slightly acidic (pH ≈ 5.6) due to dissolved carbon dioxide, which reacts with water to give carbonic acid, a weak acid.
The English chemist Robert Angus Smith is generally credited with coining the phrase acid rain in 1872 to describe the increased acidity of the rain in British industrial centers (such as Manchester), which was apparently caused by the unbridled excesses of the early Industrial Revolution, although the connection was not yet understood. At that time, there was no good way to measure hydrogen ion concentrations, so it is difficult to know the actual pH of the rain observed by Smith. Typical pH values for rain in the continental United States now range from 4 to 4.5, with values as low as 2.0 reported for areas such as Los Angeles. Recall that rain with a pH of 2 is comparable in acidity to lemon juice, and even “normal” rain is now as acidic as tomato juice or black coffee.
What is the source of the increased acidity in rain and snow? Chemical analysis shows the presence of large quantities of sulfate
and nitrate
ions, and a wide variety of evidence indicates that a significant fraction of these species come from nitrogen and sulfur oxides produced during the combustion of fossil fuels. The precursors, or chemical forerunners, of acid rain formation can also result from natural sources, such as volcanoes and decaying vegetation, but as just stated, the man-made sources, primarily emissions of sulfur dioxide (SO2) and nitrogen oxides (NOx) resulting from fossil fuel combustion are a growing contributor that can be regulated and limited (Figure 1). When sulfur dioxide and nitrogen oxides are released from power plants and other sources, prevailing winds blow these compounds across state and national borders, sometimes over hundreds of miles. Acid rain occurs when these gases react in the atmosphere with water, oxygen, and other chemicals to form various acidic compounds.
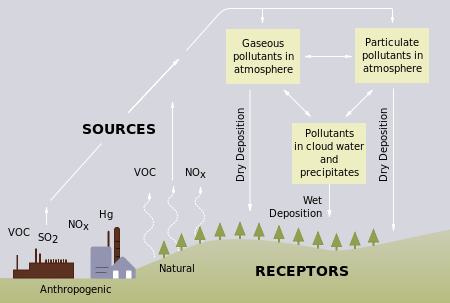
Specifically, nitric acid is the ultimate result of the interaction of nitric oxide with rain. First, at the high temperatures found in both internal combustion engines and lightning discharges, molecular nitrogen and molecular oxygen react to give nitric oxide. Nitric oxide then reacts rapidly with excess oxygen to give nitrogen dioxide, the compound responsible for the brown color of smog. When nitrogen dioxide dissolves in water, it forms a 1:1 mixture of nitrous acid and nitric acid, and then molecular oxygen eventually oxidizes nitrous acid to nitric acid, thus nitric acid (HNO3) is the ultimate end product of the reaction.
Large amounts of sulfur dioxide have always been released into the atmosphere by natural sources, such as volcanoes, forest fires, and the microbial decay of organic materials, but for most of Earth’s recorded history the natural cycling of sulfur from the atmosphere into oceans and rocks kept the acidity of rain and snow in check. Unfortunately, the burning of fossil fuels seems to have tipped the balance. Many coals contain as much as 5%–6% pyrite
by mass, and fuel oils typically contain at least 0.5% sulfur by mass. Since the mid-19th century, these fuels have been burned on a huge scale to supply the energy needs of our modern industrial society, releasing tens of millions of tons of additional
into the atmosphere annually. In addition, roasting sulfide ores to obtain metals such as zinc and copper produces large amounts of sulfur dioxide. Regardless of the source, the
dissolves in rainwater to give sulfurous acid, which is eventually oxidized by oxygen to sulfuric acid.
Concerns about the harmful effects of acid rain have led to strong pressure on industry to minimize the release of sulfur dioxide and nitric oxide.
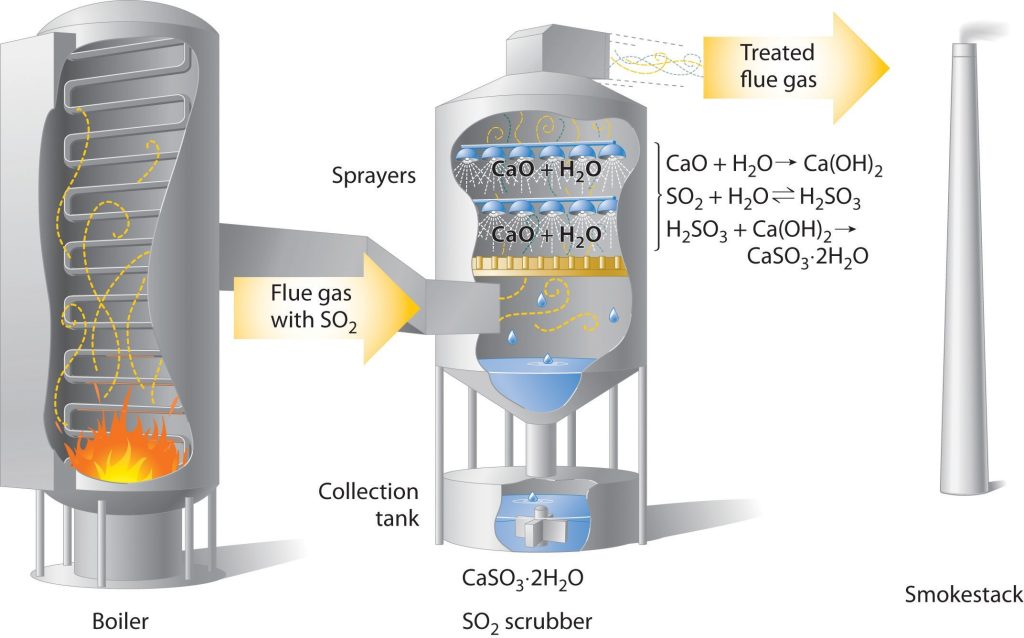
For example, coal-burning power plants now use sulfur dioxide
“scrubbers,” which trap sulfur dioxide by its reaction with lime
to produce calcium sulfite dihydrate (Figure 2).
Effects of Acid Rain
Acid rain causes acidification of lakes and streams and contributes to the damage of trees at high elevations (for example, red spruce trees above 2,000 feet) and many sensitive forest soils. In addition, acid rain accelerates the decay of building materials and paints, including irreplaceable buildings, statues, and sculptures that are part of our nation’s cultural heritage. Prior to falling to the earth, sulfur dioxide (SO2) and nitrogen oxide (NOx) gases and their particulate matter derivatives—sulfates and nitrates—contribute to visibility degradation and harm public health.
The ecological effects of acid rain are most clearly seen in the aquatic, or water, environments, such as streams, lakes, and marshes. Most lakes and streams have a pH between 6 and 8, although some lakes are naturally acidic even without the effects of acid rain. Acid rain primarily affects sensitive bodies of water, which are located in watersheds whose soils have a limited ability to neutralize acidic compounds (called “buffering capacity”). Lakes and streams become acidic (i.e., the pH value goes down) when the water itself and its surrounding soil cannot buffer the acid rain enough to neutralize it.
Biological fluids, such as blood, have a pH of 7–8. Organisms such as fish can maintain their internal pH in water that has a pH in the range of 6.5–8.5. If the external pH is too low, however, many aquatic organisms can no longer maintain their internal pH, so they die. A pH of 4 or lower is fatal for virtually all fish, most invertebrate animals, and many microorganisms. As a result of acid rain, the pH of some lakes in Europe and the United States has dropped below 4. Recent surveys suggest that up to 6% of the lakes in the Adirondack Mountains of upstate New York and 4% of the lakes in Sweden and Norway are essentially dead and contain no fish. Neither location contains large concentrations of industry, but New York lies downwind of the industrial Midwest, and Scandinavia is downwind of the most industrialized regions of western Europe. Both regions appear to have borne the brunt of the pollution produced by their upwind neighbors. One possible way to counter the effects of acid rain in isolated lakes is by adding large quantities of finely ground limestone, which would neutralize the acidity.

Acid rain also causes acidity in soils by releasing aluminum ins (Al3+). Trees and many other plants are sensitive to the presence of aluminum and other metals in groundwater. Under normal circumstances, aluminum hydroxide, which is present in some soils, is insoluble. At lower pH values, however, it dissolves. The result is increased levels of Al3+ ions in groundwater. Because the Al3+ ion is toxic to plants, high concentrations can affect plant growth. Acid rain can also weaken the leaves and roots of plants so much that the plants are unable to withstand other stresses. The combination of the two effects can cause significant damage to established forests, such as the Black Forest in Germany and the forests of the northeastern United States and Canada and other countries (Figure 4). Therefore, acid rain causes slower growth, injury, or death of forests.

Another concern of acid rain is the corrosion to human-built structures and works of art. Acid rain and the dry deposition of acidic particles contribute to the corrosion of metals (such as bronze), the deterioration of paint, and dissolution of stone (such as marble and limestone). These effects significantly reduce the societal value of buildings, bridges, cars, and cultural objects (such as statues, monuments, and tombstones) (Figure 4).
Finally, there are other direct impacts to humans worth mentioning. The sulfates and nitrates that form in the atmosphere from sulfur dioxide (SO2) and nitrogen oxides (NOx) emissions contribute to visibility impairment, meaning we cannot see as far or as clearly through the air. The pollutants also damage human health (Section 10.1). As stated at the beginning of the section, these gases interact in the atmosphere to form fine sulfate and nitrate particles that can be transported long distances by winds and inhaled deep into people’s lungs. Fine particles can also penetrate indoors. Many scientific studies have identified a relationship between elevated levels of fine particles and increased illness and premature death from heart and lung disorders, such as asthma and bronchitis.
Attribution
Essentials of Environmental Science by Kamala Doršner is licensed under CC BY 4.0. Modified from the original by Joni Baumgarten.
The Chemistry of Acid Rain by Melissa Kofoed; Shawn Miller; and Utah State University is licensed by CC BY-SA 4.0. Modified from the original by Joni Baumgarten.

