2.4 Energy Enters Ecosystems Through Photosynthesis
Section Goals:
- Understand the connection between energy and biological food webs.
- Understand photosynthesis and cellular respiration.
- Understand the link between photosynthesis, cellular respiration, and climate change.
Solar Energy Drives Ecosystems
All cells perform cellular respiration, which runs on the chemical energy. The fundamental unit of chemical energy is found in carbohydrate molecules, and the majority of carbohydrate molecules are produced by one process: photosynthesis. The energy stored in the bonds to hold carbohydrate molecules together is released when an organism breaks down food. Cells then use this chemical energy to perform work, such as movement. The energy that is harnessed from photosynthesis enters the ecosystems of our planet continuously and is transferred from one organism to another. Therefore, directly or indirectly, the process of photosynthesis provides most of the energy required by living things on Earth. Photosynthesis also results in the release of oxygen into the atmosphere. In short, to eat and breathe humans depend almost entirely on the organisms that carry out photosynthesis.

Some organisms can carry out photosynthesis, whereas others cannot. An autotroph is an organism that can produce its own food. The Greek roots of the word autotroph mean “self” (auto) “feeder” (troph). Plants are the best-known autotrophs, but others exist, including certain types of bacteria and algae (Figure 1). Oceanic algae contribute enormous quantities of food and oxygen to global food chains. More specifically, plants are photoautotrophs, a type of autotroph that uses sunlight and carbon from carbon dioxide to synthesize chemical energy in the form of carbohydrates. All organisms carrying out photosynthesis require sunlight.

Heterotrophs are organisms that obtain energy and carbon from food by consuming other organisms. The Greek roots of the word heterotroph mean “other” (hetero) “feeder” (troph), meaning that their food comes from other organisms. Even if the organism being consumed is another animal, it traces its stored energy back to autotrophs and the process of photosynthesis. Humans are heterotrophs, as are all animals and fungi. Heterotrophs depend on autotrophs, either directly or indirectly. For example, a deer obtains energy by eating plants. A wolf eating a deer obtains energy that originally came from the plants eaten by that deer (Figure 2). Using this reasoning, all food eaten by humans can be traced back to autotrophs that carry out photosynthesis.
Summary of Photosynthesis
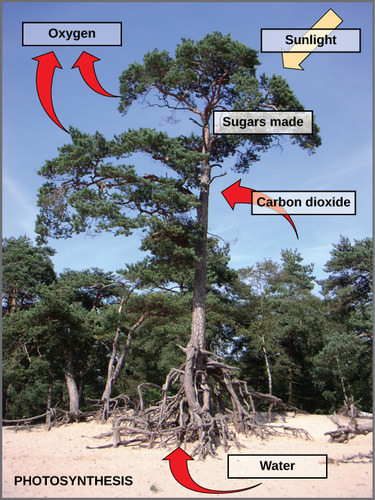
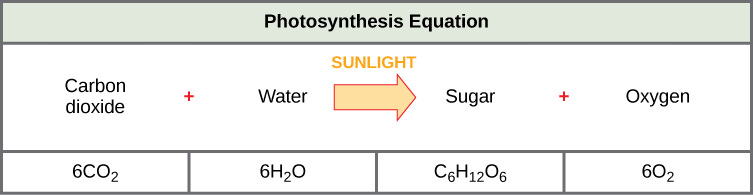
Photosynthesis requires sunlight, carbon dioxide, and water as starting reactants (Figure 3). After the process is complete, photosynthesis releases oxygen and produces carbohydrate molecules, most commonly glucose. These sugar molecules contain the energy that living things need to survive. The complex reactions of photosynthesis can be summarized by the chemical equation shown in Figure 4 below.
Although the equation looks simple, the many steps that take place during photosynthesis are actually quite complex. In plants, photosynthesis takes place primarily in the chloroplasts of leaves. Chloroplasts have a double (inner and outer) membrane. Within the chloroplast is a third membrane that forms stacked, disc-shaped structures called thylakoids. Embedded in the thylakoid membrane are molecules of chlorophyll, a pigment (a molecule that absorbs light) through which the entire process of photosynthesis begins.
The Two Parts of Photosynthesis
Photosynthesis takes place in two stages: the light-dependent reactions and the Calvin cycle. In the light-dependent reactions chlorophyll absorbs energy from sunlight and then converts it into chemical energy with the aid of water. The light-dependent reactions release oxygen as a byproduct from the splitting of water. In the Calvin cycle, the chemical energy derived from the light-dependent reactions drives both the capture of carbon in carbon dioxide molecules and the subsequent assembly of sugar molecules.
The Global Significance of Photosynthesis
The process of photosynthesis is crucially important to the biosphere for the following reasons:
- It creates O2. The molecular oxygen in Earth’s atmosphere was created by photosynthetic organisms; without photosynthesis there would be no O2 to support cellular respiration needed by complex, multicellular life. Photosynthetic bacteria were likely the first organisms to perform photosynthesis, dating back 2-3 billion years ago. Thanks to their activity, and a diversity of present-day photosynthesizing organisms, Earth’s atmosphere is currently about 21% O2. Also, this O2 is vital for the creation of the ozone layer (see Section 10.2), which protects life from harmful ultraviolet radiation emitted by the sun. Ozone (O3) is created from the breakdown and reassembly of O2.
- It provides energy for nearly all ecosystems. By transforming light energy into chemical energy, photosynthesis provides the energy used by organisms, whether those organisms are plants, grasshoppers, wolves, or fungi. Though photosynthesis captures only about 1% of total incoming solar radiation, that is enough energy to support entire biomes! The only exceptions are found in very rare and isolated ecosystems, such as near deep sea hydrothermal vents where organisms get energy that originally came from minerals, not the sun.
- It provides the carbon needed for organic molecules. Organisms are primarily made of two things: water and organic molecules, the latter being carbon based. Through the process of carbon fixation, photosynthesis takes carbon from CO2 and converts it into sugars (which are organic). Carbon in these sugars can be re-purposed to create the other types of organic molecules that organisms need, such as lipids, proteins, and nucleic acids. For example, the carbon used to make your DNA was once CO2 used by photosynthetic organisms (see Section 3.1 for more information on food webs).
Cellular respiration
Carbohydrates are storage molecules for energy in all living things. Living things access energy by breaking down carbohydrate molecules. This fundamental process is cellular respiration. You may wonder: if plants make carbohydrate molecules, do they also need to break them down? Yes! Carbohydrates are much more stable and efficient reservoirs for chemical energy and it the way energy is transferred between cells. ATP is the form of chemical energy that can be used locally to power chemical reactions within a cell. Photosynthetic organisms must also carry out the reactions of respiration to harvest the energy that they have stored in carbohydrates and convert it into ATP; plants have mitochondria in addition to chloroplasts.
Most importantly, notice that the overall reaction for photosynthesis:
6CO2 + 6H2O → C6H12O6 + 6O2
is the reverse of the overall reaction for cellular respiration:
6O2 + C6H12O6 → 6CO2 + 6H2O
Photosynthesis produces oxygen as a byproduct, and respiration produces carbon dioxide as a byproduct. In nature, there is no such thing as waste. Every single atom of matter is conserved, recycling indefinitely. Substances change form or move from one type of molecule to another, but never disappear. CO2 is no more a form of waste produced by respiration than oxygen is a waste product of photosynthesis. Both are byproducts of reactions that move on to other reactions. Photosynthesis absorbs energy to build carbohydrates in chloroplasts, and aerobic cellular respiration releases energy by using oxygen to break down carbohydrates. Photosynthesis and cellular respiration function in a biological cycle, allowing organisms to access life-sustaining energy that originates millions of miles away in a star.
Carbon dioxide, photosynthesis, and climate change
This concept will be revisited in more detail in future sections, but the key point is highlighted here:
All life produces carbon dioxide as a result of living (via cellular respiration), and requires carbon to build body tissue. Photosynthesis turns carbon dioxide into organic carbon molecules. This balance and feedback between photosynthesis and cellular respiration is a crucial part of the overall geochemical cycles that allow the earth to support life. Photosynthesis is the opposite of cellular respiration, and vice versa. It is undeniable that global carbon dioxide concentrations are rising. Thus, as a natural opposite reaction to cellular respiration, photosynthesis is a buffer to climate change caused by rising carbon dioxide levels.
Attribution
Essentials of Environmental Science by Kamala Doršner is licensed under CC BY 4.0. Modified from the original by Matthew R. Fisher and Joni Baumgarten.

