Vitamins and Minerals as Antioxidants
What Are Antioxidants, and Why Do We Need Them?
Recall from Unit 1 that atoms are composed of a nucleus, which contains neutrons and protons, and electrons, which orbit the nucleus. Atoms are most stable when they have an even number of electrons, so that they can orbit the nucleus in pairs.
An atom or group of atoms with an unpaired electron is called a free radical. Free radicals are inherently unstable and highly reactive. They steal electrons from other molecules in order to stabilize themselves, but in doing so, they create additional free radicals. This electron-grabbing is called oxidation and can set up a chain reaction, creating new free radicals and damaging important molecules along the way, similar to how one falling domino can bring down countless more.
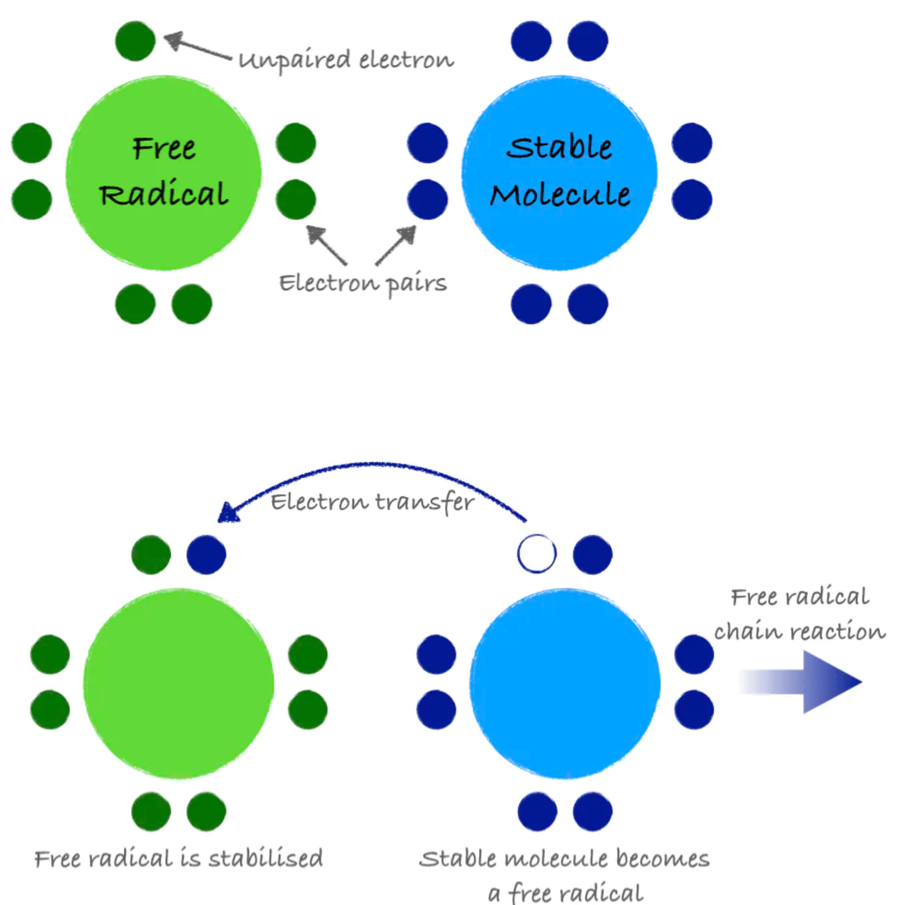
Figure 8.12. A free radical is a molecule with an unpaired electron. It can steal electrons from a stable molecule, creating a new free radical and initiating a chain reaction.
Antioxidants are molecules that can donate an electron to stabilize and neutralize free radicals. Like a domino that refuses to fall, an antioxidant can stop the free radical chain reaction in its tracks. In donating an electron, the antioxidant itself becomes a free radical. However, antioxidants are special in that they are not very reactive themselves and have processes for quick stabilization.
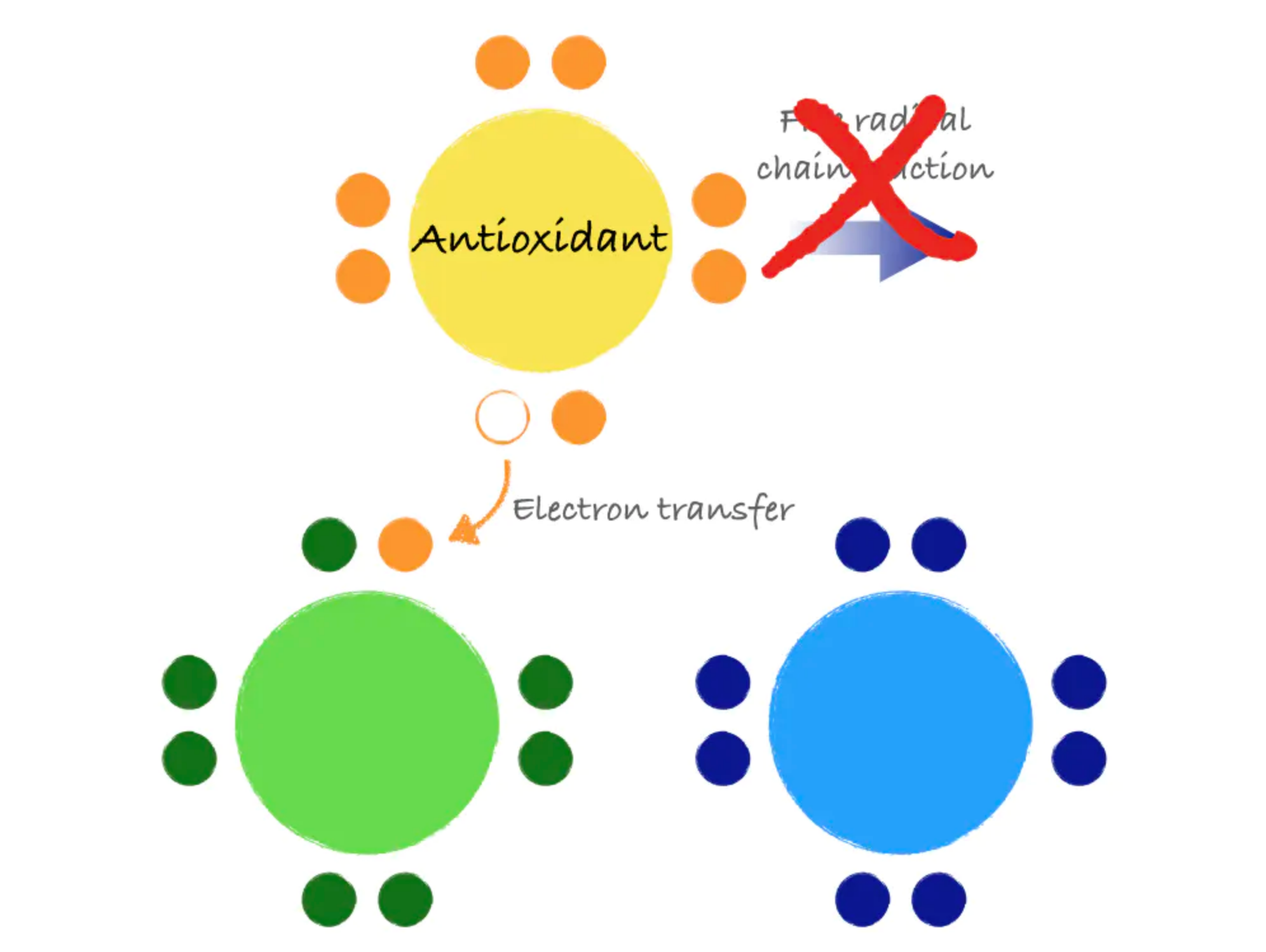
Figure 8.13. Antioxidants stabilize free radicals by donating electrons, preventing the chain reaction that can create more free radicals.
Some antioxidants are produced by the body, and some are consumed in the diet. Examples of dietary antioxidants include vitamins C and E, the mineral selenium, and phytochemicals, such as beta-carotene. We’ll focus on vitamins C and E and selenium on this page, and we’ll discuss beta-carotene on the next page.
Free radicals are a natural byproduct of metabolic reactions and of exercise, and it’s normal to have low levels of free radicals in the body. In fact, free radicals play a role in normal functioning of the body, including the ability to fight off pathogens and to send signals from one cell to another. And with enough antioxidants present, free radicals can be kept in check so that they aren’t dangerous.1
However, too many free radicals and not enough protection from antioxidants creates a situation called oxidative stress. Free radical-induced damage, when left unrepaired, destroys lipids, proteins, RNA, and DNA, and can contribute to disease. Oxidative stress has been implicated as a contributing factor to cancer, cardiovascular disease, arthritis, diabetes, kidney disease, Alzheimer’s disease, Parkinson’s disease, and eye diseases such as cataracts.2
Substances and energy sources from the environment can add to or accelerate the production of free radicals within the body. Exposure to UV radiation (e.g., from sunlight), air pollution, tobacco smoke, heavy metals, ionizing radiation, asbestos, and other toxic chemicals increase the amount of free radicals in the body. They do so by being free radicals themselves or by adding energy that provokes electrons to move between atoms. Excessive exposure to environmental sources of free radicals can contribute to disease by overwhelming the free radical detoxifying systems and those processes involved in repairing oxidative damage.
Oxidative stress is associated with the development of chronic diseases, and eating a diet rich in antioxidant-containing foods like fruits, vegetables, and whole grains seems to protect against many of these same diseases. It was thus natural for researchers to hypothesize that taking antioxidants in supplement form might also offer protection from these diseases. However, several decades of research investigating this hypothesis have revealed disappointing results. Not only have these studies shown that antioxidant supplements generally aren’t beneficial, some have shown that they can cause health risks. For example, high doses of beta-carotene increased the risk of lung cancer in smokers, and high doses of vitamin E increased the risk of prostate cancer in men. Antioxidant supplements may also interact with other medications, further emphasizing the importance of talking with your doctor before taking supplements.2
Researchers aren’t sure why some antioxidant supplements have turned out to be dangerous. It may come back to the fact that free radicals play important roles in the body, and adding high doses of antioxidant supplements overwhelms the normal balance of free radicals and does more harm than good. It may also be that the benefits of eating antioxidant-rich foods come not just from the antioxidants but from the entire package of nutrients, like fiber and phytochemicals in the whole foods, a combination that simply can’t be replicated in a pill. Regardless, you can obtain adequate levels of dietary antioxidants simply by eating a healthy diet.1,2
Let’s take a closer look at several of the most important dietary antioxidants: vitamin E, vitamin C, and selenium.
Vitamin E
When we talk about vitamin E, we’re actually referring to 8 chemically similar substances, of which alpha-tocopherol appears to be the most potent antioxidant. Because vitamin E is fat-soluble, its antioxidant capacity is especially important to lipids, including those in cell membranes and lipoproteins. For example, free radicals can oxidize LDL cholesterol (stealing an electron from it), and it is this damaged LDL that lodges in blood vessels and forms the fatty plaques characteristic of atherosclerosis, increasing the risk of heart attack, stroke, and other complications of cardiovascular disease.
After alpha-tocopherol interacts with a free radical it is no longer capable of acting as an antioxidant unless it is enzymatically regenerated. Vitamin C helps to regenerate some of the alpha-tocopherol, but the remainder is eliminated from the body. Therefore, to maintain vitamin E levels, you ingest it as part of your diet.
In addition to its antioxidant functions, vitamin E, mainly as alpha-tocopherol, plays a role in the immune system, regulation of gene expression, and cell signaling. It also enhances the dilation of blood vessels and inhibits blood clot formation.
Food Sources of Vitamin E
Excellent dietary sources of vitamin E include nuts, seeds, and vegetable oils, with additional amounts provided by green leafy vegetables and fortified cereals.3
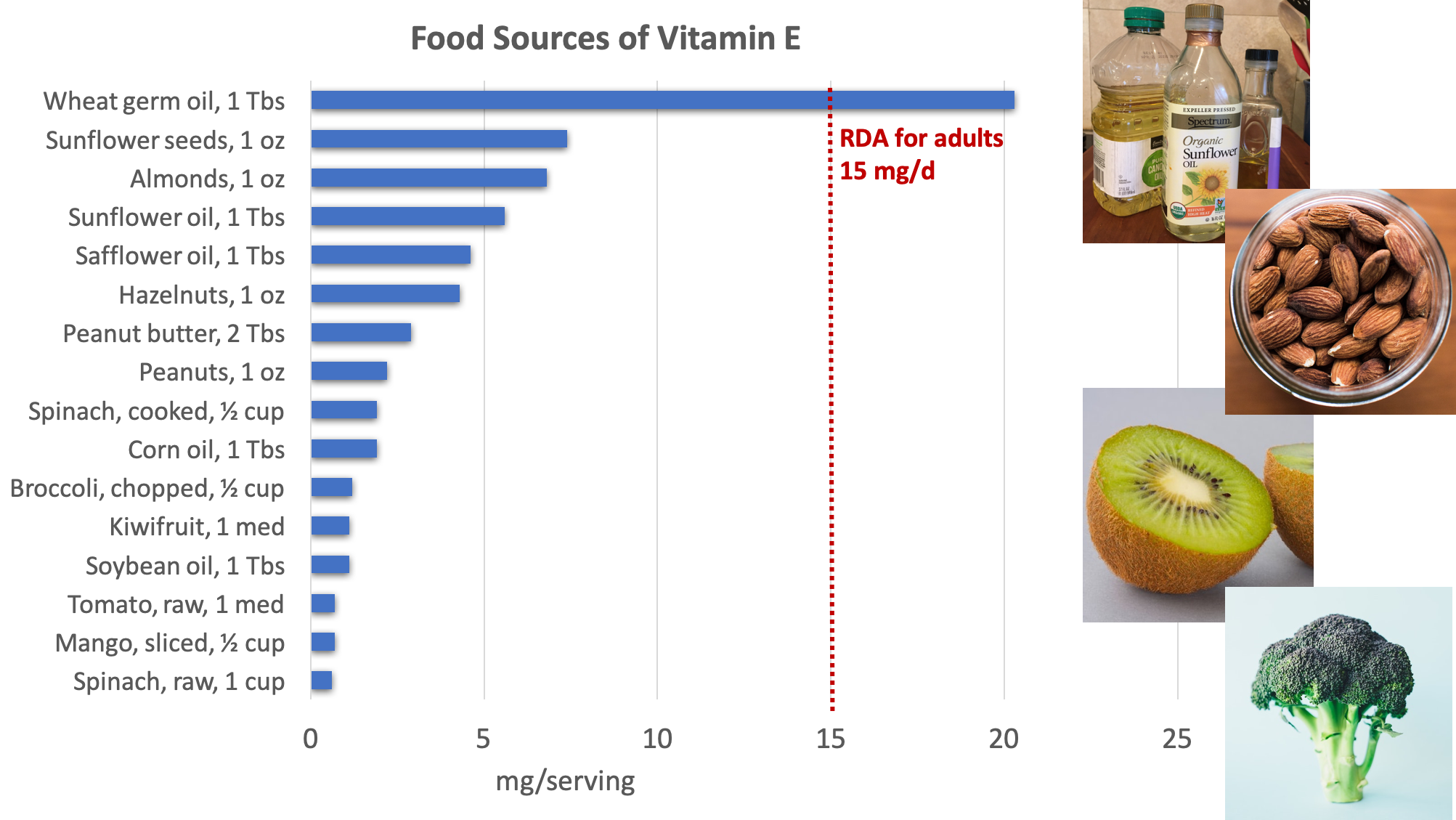
Figure 8.14. Dietary sources of vitamin E. Source: NIH Office of Dietary Supplements
Surveys of Americans’ diets often find that they provide less than the RDA for vitamin E. However, these studies may underestimate the amount of vitamin E in the diet because they don’t fully account for vegetable oils in the diet, as these are rich sources of vitamin E. Vitamin E can be destroyed at high temperatures, especially when reheated repeatedly, so oils used in deep frying are not good sources of the vitamin.
Vitamin E Deficiency and Toxicity
Outright vitamin E deficiency with obvious deficiency symptoms is very rare in healthy people. It most often occurs in people with an underlying disorder that impairs the digestion and absorption of fat. Symptoms of vitamin E deficiency include nerve and muscle damage, vision problems, and a weakened immune system.3
Studies have not found any risks of consuming vitamin E in foods. The UL for vitamin E is set at 1,000 mg for adults, far above the RDA of 15 mg and far higher than could naturally be obtained from food. These amounts are available in supplement form, however. As mentioned, high-dose vitamin E supplements were shown to increase the risk of prostate cancer in men. Other studies have found that high-dose vitamin E supplements are associated with an increased risk of hemorrhage, stroke, and death.
Vitamin C
Vitamin C, also called ascorbic acid, is a water-soluble vitamin essential in the diet for humans. Interestingly, most other mammals can readily synthesize vitamin C and don’t require it in their diets. Vitamin C’s ability to easily donate electrons makes it a highly effective antioxidant. Since it is water-soluble, it acts both inside and outside cells to protect molecules in aqueous environments. Vitamin C also plays a vital role in regenerating vitamin E after it has acted as an antioxidant, allowing it to be recycled and used again.
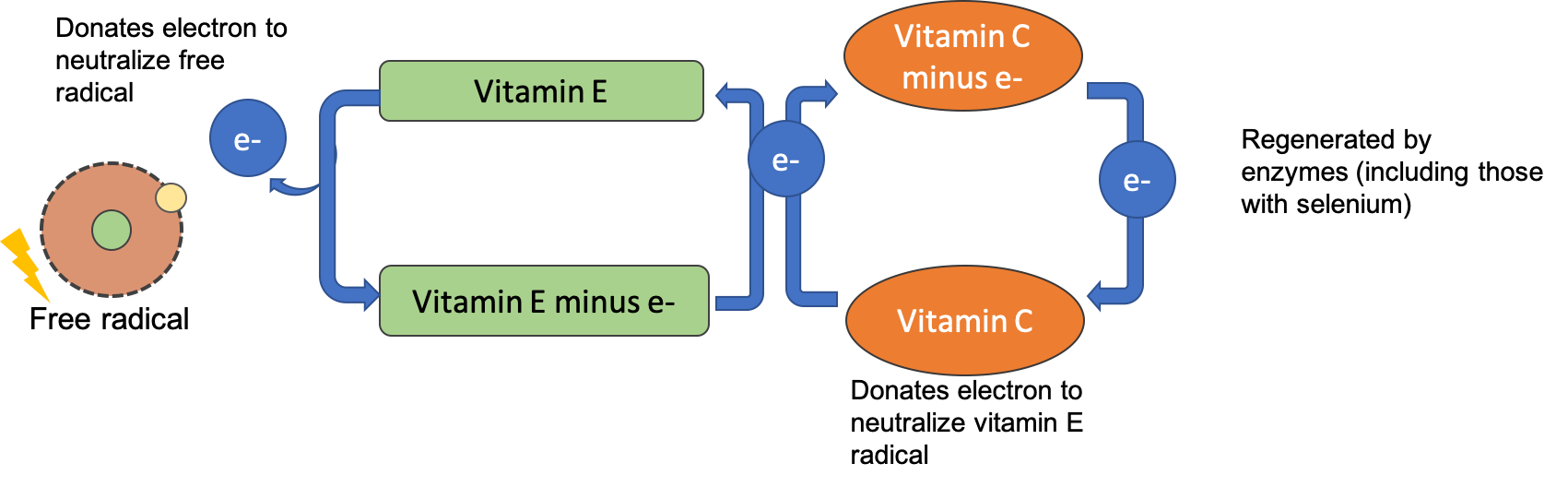
Figure 8.15. After vitamin E donates an electron to neutralize a free radical, it can be regenerated by an electron from vitamin C. Vitamin C is then regenerated by antioxidant enzymes.
In addition to its role as an antioxidant, vitamin C is a required part of several enzymes involved in the synthesis of collagen, a protein important to the strength and structure of muscles, bones, tendons, ligaments, connective tissue, and skin. Vitamin C is also required to synthesize neurotransmitters important for signaling in the brain, some hormones, and amino acids. It also plays a role in immune function and improves the absorption of dietary iron.
The body’s vitamin C status is tightly controlled to maintain steady tissue and plasma concentrations. This means that if you consume high doses of vitamin C, you’ll absorb less from the intestine and excrete more in urine to prevent excessive concentrations in the body. Vitamin C is not stored in any significant amount in the body, but once it has reduced a free radical, it is very effectively regenerated and therefore can exist in the body as a functioning antioxidant for many weeks.
Food Sources of Vitamin C
Fruits and vegetables are great sources of vitamin C. Some of the best sources include bell pepper, citrus, broccoli, strawberries, Brussels sprouts, and cantaloupe.4
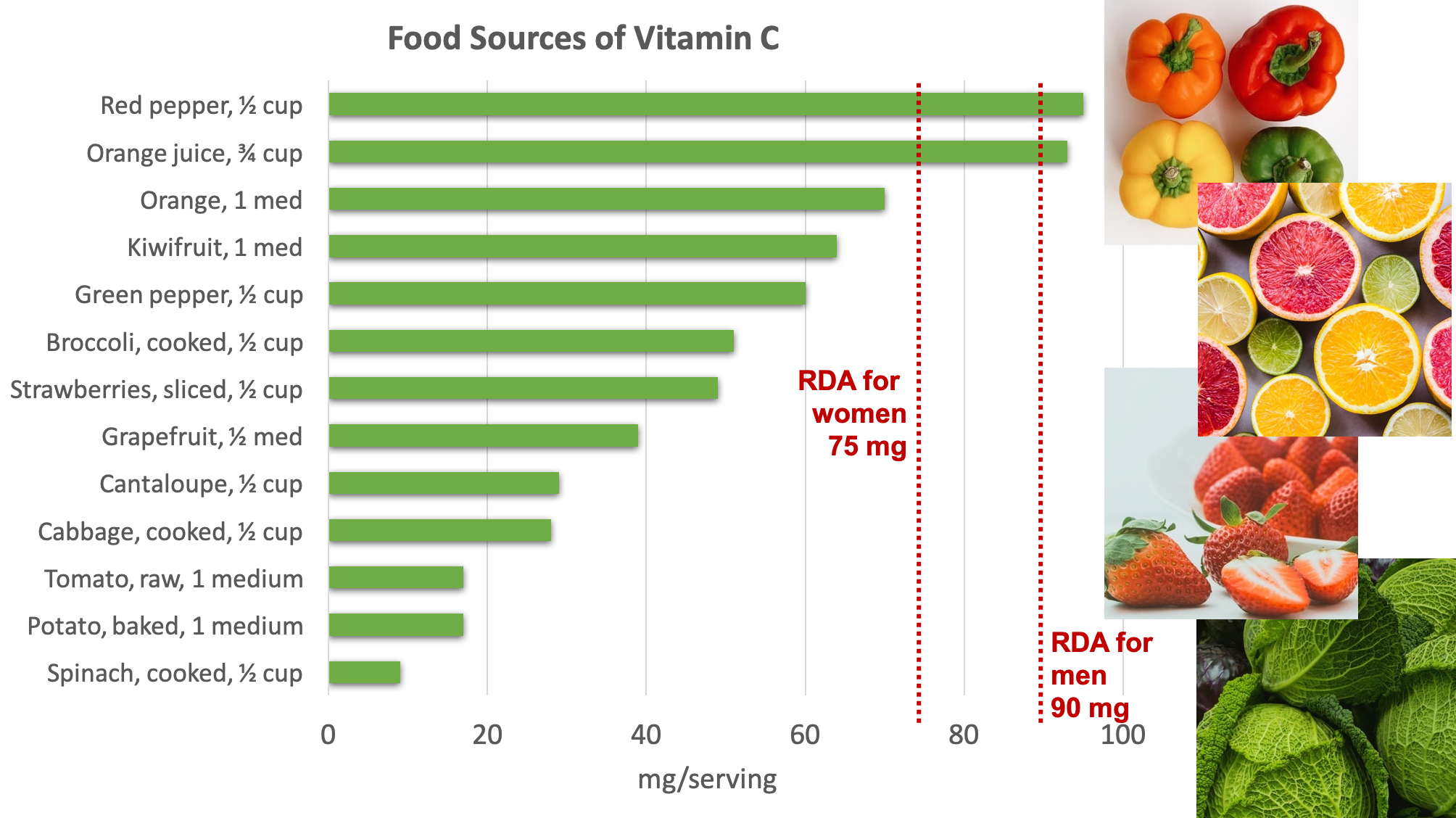
Figure 8.16. Dietary sources of vitamin C. Source: NIH Office of Dietary Supplements
Because vitamin C is water-soluble, it leaches away from foods considerably during cooking, freezing, thawing, and canning. Up to 50% of vitamin C can be boiled away. Therefore, eating fruits and vegetables raw or lightly steamed maximizes the vitamin C value of these foods.
Vitamin C Deficiency and Toxicity
The classic condition caused by vitamin C deficiency is scurvy. The signs and symptoms of scurvy include skin disorders, bleeding gums, joint pain, and weakness—all of which may be related to vitamin C’s role in collagen synthesis. Additional symptoms of scurvy include abnormally-thickened skin, fatigue, depression, iron deficiency anemia, and increased susceptibility to infections.
In the past, scurvy was common among sailors on long ocean voyages, whose diets were completely lacking in fruits and vegetables for many months. In the mid-1700s, British Navy surgeon Sir James Lind’s experiments revealed that citrus fruits and juices could prevent scurvy in sailors. British sailors were often referred to as “limeys,” as they carried sacks of limes onto ships to prevent scurvy. It was not until 1932 that scientists showed that vitamin C was the essential nutrient involved in this cure.
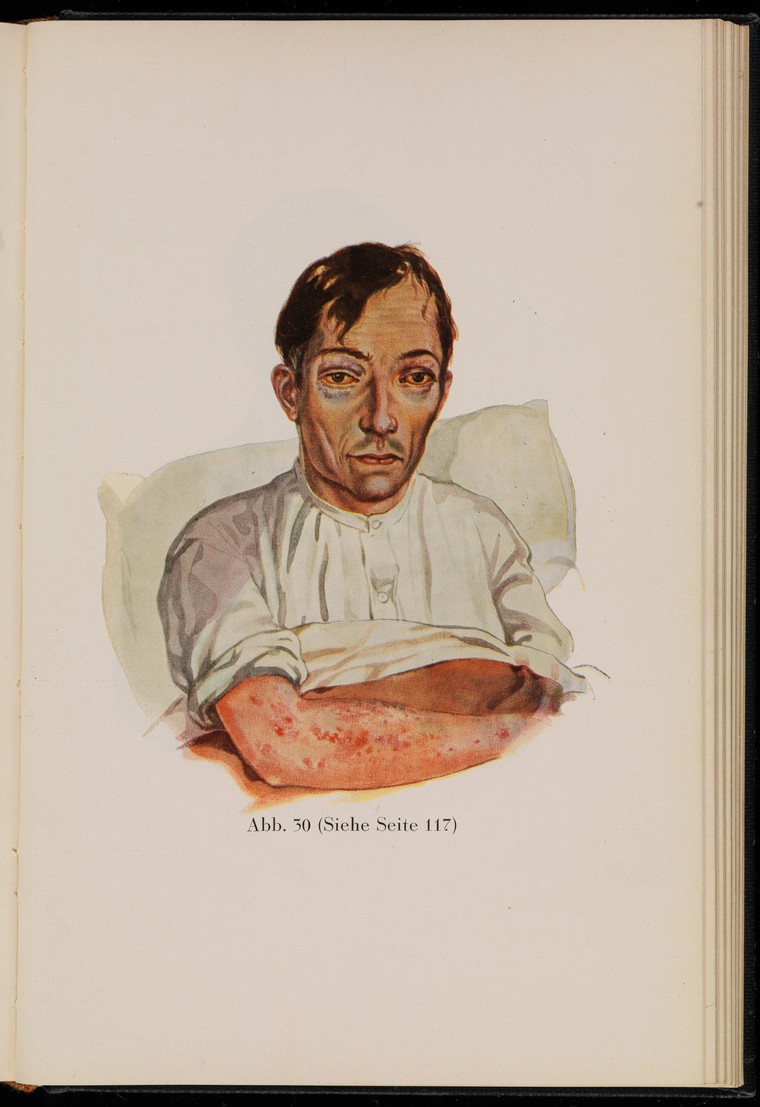
Figure 8.17. This drawing of a sailor with scurvy, from the 1929 edition of “Kranken-Physiognomik” by von K. H. Baumgärtner, shows common effects of the disease, including sunken eyes and skin lesions.
Scurvy is prevented by even a low intake of fruits and vegetables, and it takes at least a month of consuming very little or no vitamin C for scurvy symptoms to develop. Thus, scurvy is rare in developed countries today. Diet surveys show that most Americans meet the RDA for vitamin C. When vitamin C deficiency occurs today, it is in people who consume very limited food variety, such as those with mental illness, people who abuse alcohol or drugs, people on very restrictive diets, and impoverished people with limited fruit and vegetable access. People who smoke also require more vitamin C to counter the free radicals generated by smoking.
The risk of vitamin C toxicity from foods is essentially nonexistent, because the body can adjust intestinal absorption and urinary excretion to maintain a healthy vitamin C level. However, high doses of vitamin C from supplements have been reported to cause numerous problems, including gastrointestinal upset and diarrhea. To prevent these discomforts, the UL for vitamin C is set at 2,000 milligrams per day for adults, more than twenty times the RDA.
At very high doses in combination with iron, vitamin C has sometimes been found to increase oxidative stress, reaffirming that getting your antioxidants from foods is better than getting them from supplements. There is also some evidence that taking vitamin C supplements at high doses increases the likelihood of developing kidney stones; however, this effect is most often observed in people that already have multiple risk factors for kidney stones.
Can Vitamin C Supplements Prevent the Common Cold?
Many people believe that taking a vitamin C supplement can prevent the common cold or decrease its symptoms. This idea was popularized by Linus Pauling in the 1970s, and it’s continuously promoted today in the form of over-the-counter supplements such as Emergen-C and Airborne. These typically contain doses in the range of 1000 mg of vitamin C, far higher than normal levels of vitamin C in the diet and enough to reach the UL of 2000 mg if a person takes two doses per day.

Do these high-dose vitamin C supplements do anything to prevent or treat the misery of the common cold? A systematic review and meta-analysis published in 2013 by the Cochrane Collaboration summarized the results of 29 studies conducted on this question. The review concluded that for most people, these supplements don’t prevent the common cold but can reduce the duration of symptoms by 8% in adults and 14% in children—amounting to a day or two of relief—but only if they’re taken consistently every day and before cold symptoms begin. If taken after the onset of symptoms, a vitamin C supplement does not seem to reduce the duration or severity of symptoms. Some research shows that vitamin C supplements may be more effective in cold prevention in athletes and those in extreme physical conditions, such as marathon runners, endurance skiers, and soldiers.5
Selenium
Selenium is an essential trace mineral. It is part of the structure of at least 25 proteins in the body, with functions in reproduction, thyroid hormone metabolism, DNA synthesis, and antioxidant and immune protection.6 As part of antioxidant enzymes, selenium helps to regenerate other antioxidants, including vitamin C. These enzymes also protect lipids from free radicals, and, in doing so, spare vitamin E. This illustrates how antioxidants work together to protect the body against free radical-induced damage.
Food Sources of Selenium
Organ meats, muscle meats, and seafood have the highest selenium content. Grains and some nuts contain selenium when grown in selenium-containing soils. The selenium content of the soils used to grow animal feed can also affect the selenium content of animal products.
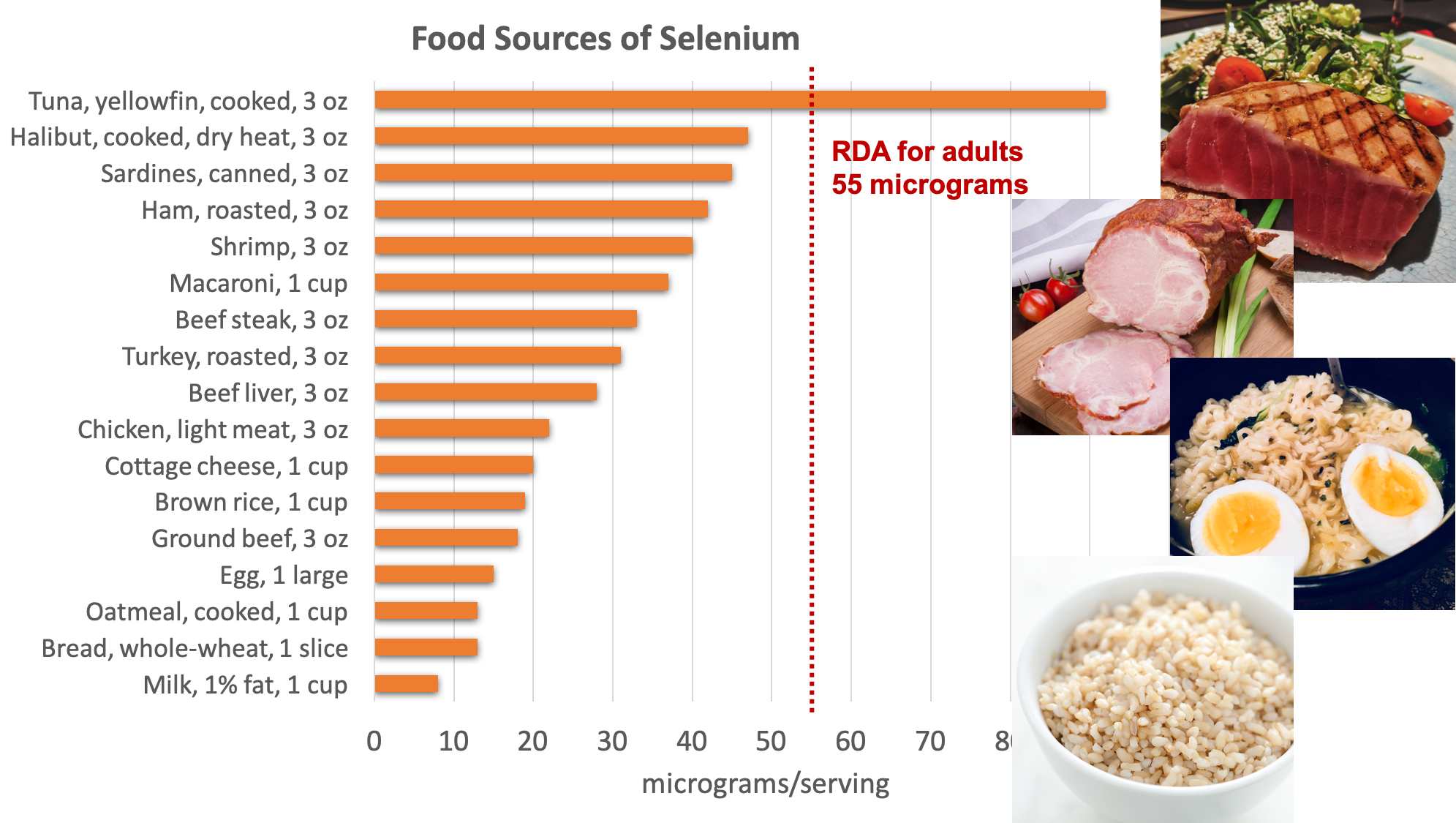
Figure 8.18. Dietary sources of selenium. Source: NIH Office of Dietary Supplements
Selenium Deficiency and Toxicity
Selenium deficiency is very rare in the United States and other developed countries. Worldwide, people with a primarily vegetarian diet in areas with low soil selenium levels, including parts of China and Europe, may be at risk for selenium deficiency.6
Chronic exposure to foods grown in soils containing high levels of selenium (above the UL of 400 micrograms per day) can cause brittle hair and nails, gastrointestinal discomfort, skin rashes, halitosis, fatigue, and irritability. Brazil nuts contain very high levels of selenium, so if eaten regularly could cause selenium toxicity. Selenium at doses several thousand times the RDA can cause acute toxicity, and when ingested in gram quantities, can be fatal.
Self-Check:
Attributions:
- Zimmerman, M., & Snow, B. Nutrients Important as Antioxidants. In An Introduction to Nutrition (v. 1.0). https://2012books.lardbucket.org/books/an-introduction-to-nutrition/index.html, CC BY-NC-SA 3.0
References:
- 1Adcock, J. (2018, January 10). What are antioxidants? And are they truly good for us? The Conversation. https://theconversation.com/what-are-antioxidants-and-are-they-truly-good-for-us-86062
- 2National Center for Complementary and Integrative Health. (n.d.). Antioxidants: In Depth. Retrieved April 2, 2020, from https://www.nccih.nih.gov/health/antioxidants-in-depth
- 3National Institutes of Health Office of Dietary Supplements. (n.d.). Vitamin E – Health Professional Fact Sheet. Retrieved April 2, 2020, from https://ods.od.nih.gov/factsheets/VitaminE-HealthProfessional/
- 4National Institutes of Health Office of Dietary Supplements. (n.d.). Vitamin C – Health Professional Fact Sheet. Retrieved April 2, 2020, from https://ods.od.nih.gov/factsheets/VitaminC-HealthProfessional/
- 5Hemila, H., & Chalker, E. (2013). Vitamin C for preventing and treating the common cold. Cochrane Database of Systematic Reviews, CD000980. https://www.ncbi.nlm.nih.gov/pubmed/23440782
- 6National Institutes of Health Office of Dietary Supplements. (n.d.). Selenium- Health Professional Fact Sheet. Retrieved April 2, 2020, from https://ods.od.nih.gov/factsheets/Selenium-HealthProfessional/
Image Credits:
- Figure 8.12. “Free radical chain reaction” by Jacqui Adcock is licensed under CC BY-NC-ND 4.0
- Figure 8.13. “Antioxidant action” by Jacqui Adcock is licensed under CC BY-NC-ND 4.0
- Figure 8.14. “Food sources of Vitamin E” by Alice Callahan is licensed under CC BY 4.0, with images from top to bottom by Alice Callahan, CC BY 4.0; and Remi Yuan, Annie Spratt, and engin akyurt on Unsplash (license information)
- Figure 8.15. “Vitamin E/vitamin C antioxidant cycle” by Alice Callahan is licensed under CC BY 4.0
- Figure 8.16. “Food sources of Vitamin C” by Alice Callahan is licensed under CC BY 4.0, with images from top to bottom by Bruna Branco, Irene Kredenets, Kyaw Tun, and Forest Diver on Unsplash (license information)
- Figure 8.17. “38 year old man suffering from scurvy” by von K. H. Baumgärtner, Welcomme Library is licensed under CC BY 4.0
- “Emergen-C supplement photo” by Mike Mozart is licensed under CC BY 2.0
- Figure 8.18. “Food sources of selenium” by Alice Callahan is licensed under CC BY 4.0, with images from top to bottom by sunorwind, Сергей Орловский, Cake Tuxedo on Unsplash (license information), and “par cooked brown rice” by jules is licensed under CC BY-NC 2.0
An atom or group of atoms with an unpaired electron; they are unstable, highly reactive, and can be damaging in excess.
The loss of electrons during a reaction.
Molecules that can donate an electron to stabilize and neutralize free radicals.
Free radical-induced damage that can contribute to disease.
A protein important to the strength and structure of muscles, bones, tendons, ligaments, connective tissue, and skin.
A disease caused by vitamin C deficiency.

