The Scientific Method
Similar to the method by which a police detective investigates a crime, nutritional scientists discover the health effects of food and nutrients by first making an observation and posing a question that they’d like to answer. Then they formulate a hypothesis, test their hypothesis through experiments, and finally interpret the results. After analyzing additional evidence from multiple sources, they may form a conclusion on whether the food suspect fits the claim. This organized process of inquiry used in forensic science, nutritional science, and every other science, is called the scientific method.
The basis of what we know about nutrition is derived from research, and the scientific method underlies how research is conducted. The steps of the scientific method include:
1. Observation/Question: Researchers first make an observation and come up with a research question to investigate.
2. Hypothesis: The researchers formulate a hypothesis, or educated guess, that would explain the observation or question and that can be tested through scientific experiments.
3. Experiment: The researchers design and conduct an experiment. A good design takes into account what has been done previously. Thus, before beginning a new study, researchers undertake a thorough review of published research in order to ensure that their work advances the field.
4. Analysis: The researchers collect and analyze data that will either support or refute the hypothesis. If the hypothesis is not supported, the researcher creates a new hypothesis and conducts a new experiment. If the hypothesis is supported, researchers will design additional experiments to try to replicate the findings or to test them in different ways.
5. Conclusion: After multiple experiments consistently support a hypothesis, researchers can offer a conclusion or theory.
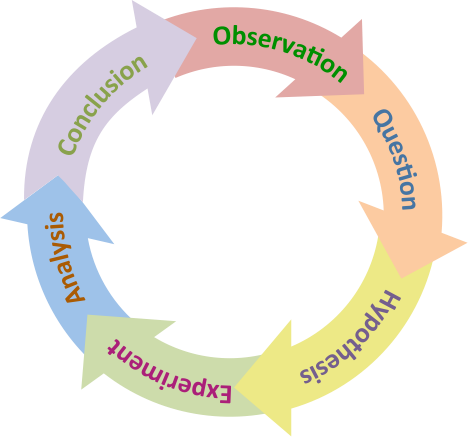
Figure 2.1. The scientific method is a cyclical process, because it always leads to new observations and questions.
Through the scientific method, our knowledge of science builds continuously over time. No single study is enough to fully explain any one phenomenon, particularly in an area as complex as nutrition. Even experiments that go exactly as expected lead us to new questions to investigate. Science is also filled with surprises, both big and small. Experiments may not yield the expected results, but unexpected results can lead to new and important questions. And because scientists are human, they can make mistakes along the way or fail to acknowledge or test an important variable, which is why it’s important that experiments be repeated and evaluated by other researchers along the way. In fact, scientific advances rarely occur because of one person. Scientists usually tackle research projects in teams made up of people with different types of expertise, and it generally takes multiple teams of researchers looking at the same question from different perspectives and through different approaches to reach a consensus—when scientists broadly agree on certain observations and interpretations of data.
The history of nutrition is full of fascinating examples of the scientific method at work. One such example is the discovery that the mineral iodine is an essential nutrient. This story of scientific discovery began in 1811, when French chemist Bernard Courtois was isolating a substance called saltpeter, an ingredient needed to make gunpowder for Napoleon’s army. Part of his isolation procedure involved burning seaweed, during which Courtois observed the release of an intense violet vapor, which crystallized when he exposed it to a cold surface. He sent the violet crystals to an expert on gasses, Joseph Gay-Lussac, who identified the crystal as a new element. It was named iodine after the Greek word for violet. But identifying iodine was just the beginning. The following timeline traces the scientific experiments, conducted by multiple investigators around the world, that were required to show that iodine is an essential nutrient.1,2
Early 1800s: Swiss physician Jean-Francois Coindet observed that eating seaweed was an effective cure for goiter, an enlargement of the thyroid gland in the neck. In 1813, he hypothesized that seaweed contained iodine and that he could use iodine instead of seaweed to treat his patients. He conducted an experiment by administering iodine tincture orally to his patients with goiter. The treatment worked, which Coindet interpreted as confirming his hypothesis that iodine tincture could treat goiter. 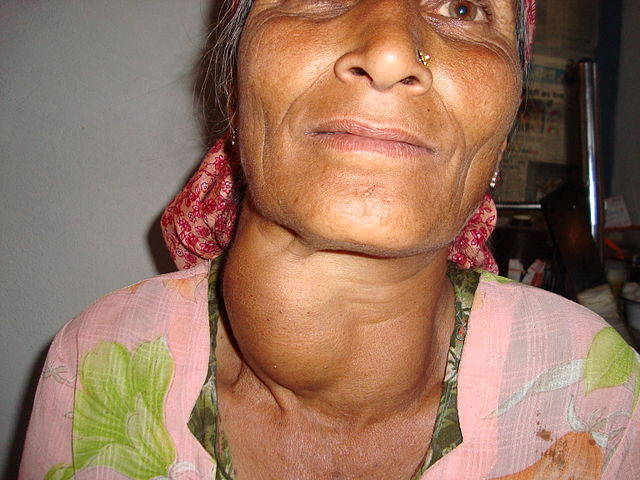
Figure 2.2. A large goiter in a woman from India.
Mid 1800s: Many other physicians contributed to the research on iodine deficiency and goiter. At the time, it was commonly believed that goiter was the result of poor hygiene, drunkenness, dampness, or contaminated water. But in 1851, French chemist Chatin hypothesized that the low levels of iodine found in food and water from certain areas far away from the ocean was the primary cause of goiter. In the late 1860s, authorities in several French villages began giving out iodine tablets and salt in an experimental effort to treat goiter. The program was effective, and 80 percent of goitrous children were cured. However, adults did not always respond well to the treatment, and because men with goiter were exempted from service in the French military, some people were opposed to treating it. Some scientists also insisted that goiter was caused by infectious disease, so iodine wasn’t yet accepted as a means of preventing it.
Early 1900s: In 1918, Swiss doctor Bayard proposed iodizing salt as a way of increasing iodine intake in people living in areas where goiter was common. Iodized salt was transported by mules to a small village at the base of the Matterhorn, where more than 75 percent of school children were goitrous. In an experimental trial, the iodized salt was given to families to use for six months. It resulted in reduced incidence of goiter in this remote population. Later, Physician David Marine conducted the first U.S. experiment of treating goiter with iodized salt in Akron, Ohio.The study, conducted on over 4,000 school children, found that iodized salt prevented goiter. Seven other studies similar to Marine’s were conducted in Italy and Switzerland, and they also demonstrated the effectiveness of iodized salt in treating goiter. In 1924, U.S. public health officials initiated the program of iodizing salt and started eliminating the scourge of goiterism. Today, more than 70 percent of American households use iodized salt, and many other countries have followed the same public health strategy to reduce the health consequences of iodine deficiency.
It took more than one hundred years — and the work of many different scientists from around the world — from iodine’s discovery as an effective treatment for goiter until public health programs recognized it as such. During that time, there were no doubt many different theories about the cause of goiter and debate about how it should be treated and prevented. Indeed, uncertainty is a part of the scientific process. Scientists must grapple with different possible explanations for their observations, and even as they may pursue one hypothesis, remain open-minded to alternative ones. Over time, as more evidence accumulates pointing toward a common conclusion, scientists reach a consensus, which the vast majority believe to be true. There is now a clear consensus that iodine deficiency causes goiter, and that iodizing salt is an effective means of preventing it.
Reporting Scientific Work
As we saw with the story of iodine research, scientists must share their findings in order for other researchers to expand and build upon their discoveries. Collaboration with other scientists when planning and conducting studies and analyzing results is important for scientific research. For this reason, communicating with peers and disseminating study results are important aspects of a scientist’s work. Scientists can share results by presenting them at a scientific meeting or conference, but this approach can reach only the select few who are present. Instead, most scientists present their results in peer-reviewed publications that are published in scientific journals.
Peer-reviewed publications are scientific papers that are reviewed by a scientist’s colleagues, or peers. These colleagues are qualified individuals, often experts in the same research area, who judge whether or not the scientist’s work is suitable for publication. The process of peer review is a quality control step; its goal is to ensure that the research described in a scientific paper is original, significant, logical, and thorough. It’s important to note that peer review doesn’t mean a study is perfect or even good. Sometimes bad studies slip through peer review, but because they’re published and other scientists read them, these are usually caught later and often retracted.
Peer review happens in other ways in science, too. When researchers apply for grants to fund their work, it is often other experts from their field that evaluate their grant proposals and help decide whether they should be funded. In a more informal way, researchers are reviewed by their peers when they present their findings at a conference.
Science is often messy and imperfect, but peer-review and publication of results are essential to its progress and ability to self-correct when people make mistakes.
VIDEO: “Peer Review in 3 Minutes” by libncsu, YouTube (May 1, 2014), 3:14 minutes.
Self-Check:
Attributions:
- “The Scientific Method” section 1.13 from Lindshield, B. L. Kansas State University Human Nutrition (FNDH 400) Flexbook. goo.gl/vOAnR, CC BY-NC-SA 4.0
- “The Broad Role of Nutritional Science,” section 1.3 from the book An Introduction to Nutrition (v. 1.0), CC BY-NC-SA 3.0
- “Reporting Scientific Work” section 5 from Jones, T.G. The Science of Biology, CC BY-NC-SA 4.0
References:
- 1Carpenter, K. J. (2005). David Marine and the Problem of Goiter. The Journal of Nutrition, 135(4), 675–680. https://doi.org/10.1093/jn/135.4.675
- 2Zimmermann, M. B. (2008). Research on Iodine Deficiency and Goiter in the 19th and Early 20th Centuries. The Journal of Nutrition, 138(11), 2060–2063. https://doi.org/10.1093/jn/138.11.2060
Images:
- Figure 2.1. “The Scientific Method” by Thebiologyprimer is in the Public Domain
- Figure 2.2. “A large goiter” by Dr. J.S.Bhandari, India is licensed under CC BY-SA 3.0
An organized process of inquiry used in nutritional science, and every other science; made up of a cyclical process of steps including observation/question, hypothesis, experiment, analysis, and conclusion.
When scientists broadly agree on certain observations and interpretations of data.
Scientific papers that are reviewed by other experts in the field, who were not directly involved in the research, before publication.

