2.1 Matter
Atoms, Molecules, & Compounds
At its most fundamental level, life is made of matter. Matter is something that occupies space and has mass. All matter is composed of elements, substances that cannot be broken down or transformed chemically into other substances. Each element is made of atoms, each with a constant number of protons and unique properties. A total of 118 elements have been defined; however, only 92 occur naturally and fewer than 30 are found in living cells. The remaining 26 elements are unstable and therefore do not exist for very long or are theoretical and have yet to be detected. Each element is designated by its chemical symbol (such as H, N, O, C, and Na), and possesses unique properties. These unique properties allow elements to combine and to bond with each other in specific ways.
An atom is the smallest component of an element that retains all of the chemical properties of that element. For example, one hydrogen atom has all of the properties of the element hydrogen, such as it exists as a gas at room temperature and it bonds with oxygen to create a water molecule. Hydrogen atoms cannot be broken down into anything smaller while still retaining the properties of hydrogen. If a hydrogen atom were broken down into subatomic particles, it would no longer have the properties of hydrogen. At the most basic level, all organisms are made of a combination of elements. They contain atoms that combine together to form molecules. In multicellular organisms, such as animals, molecules can interact to form cells that combine to form tissues, which make up organs. These combinations continue until entire multicellular organisms are formed.
All matter, whether it be a rock or an organism, is made of atoms. Often, these atoms combine to form molecules. Molecule are chemicals made from two or more atoms bonded together. Some molecules are very simple, like O2, which is comprised of just two oxygen atoms. Some molecules used by organisms, such as DNA, are made of many millions of atoms. All atoms contain protons, electrons, and neutrons (Figure 1 below). The only exception is hydrogen (H), which is made of one proton and one electron. A proton is a positively charged particle that resides in the nucleus (the core of the atom) of an atom and has a mass of 1 and a charge of +1. An electron is a negatively charged particle that travels in the space around the nucleus. In other words, it resides outside of the nucleus. It has a negligible mass and has a charge of –1. Neutrons, like protons, reside in the nucleus of an atom. They have a mass of 1 and no charge. The positive (protons) and negative (electrons) charges balance each other in a neutral atom, which has a net zero charge.
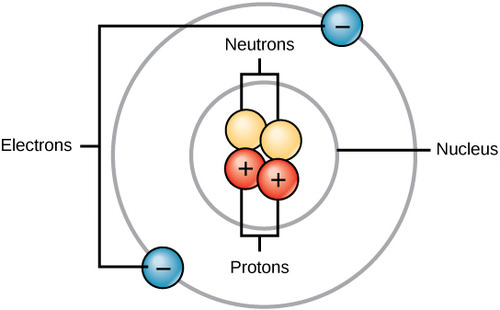
Each element contains a different number of protons and neutrons, giving it its own atomic number and mass number. The atomic number of an element is equal to the number of protons that element contains. The mass number is the number of protons plus the number of neutrons of that element. Therefore, it is possible to determine the number of neutrons by subtracting the atomic number from the mass number.
Isotopes are different forms of the same element that have the same number of protons, but a different number of neutrons. Some elements, such as carbon, potassium, and uranium, have naturally occurring isotopes. Carbon 12, the most common isotope of carbon, contains six protons and six neutrons. Therefore, it has a mass number of 12 (six protons and six neutrons) and an atomic number of 6 (which makes it carbon). Carbon 14 contains six protons and eight neutrons. Therefore, it has a mass number of 14 (six protons and eight neutrons) and an atomic number of 6, meaning it is still the element carbon. These two alternate forms of carbon are isotopes. Some isotopes are unstable and will lose protons, other subatomic particles, or energy to form more stable elements. These are called radioactive isotopes or radioisotopes.
| EVOLUTION IN ACTION |
|
Carbon dating Carbon-14 (14C) is a naturally occurring radioisotope that is created in the atmosphere by cosmic rays. This is a continuous process, so more 14C is always being created. As a living organism develops, the relative level of 14C in its body is equal to the concentration of 14C in the atmosphere. When an organism dies, it is no longer ingesting 14C, so the ratio will decline. 14C decays to 14N by a process called beta decay; it gives off energy in this slow process. After approximately 5,730 years, only one-half of the starting concentration of 14C will have been converted to 14N. The time it takes for half of the original concentration of an isotope to decay to its more stable form is called its half-life. Because the half-life of 14C is long, it is used to age formerly living objects, such as fossils. Using the ratio of the 14C concentration found in an object to the amount of 14C detected in the atmosphere, the amount of the isotope that has not yet decayed can be determined. Based on this amount, the age of the fossil can be calculated to about 50,000 years (Figure 2 below). Isotopes with longer half-lives, such as potassium-40, are used to calculate the ages of older fossils. Through the use of carbon dating, scientists can reconstruct the ecology and biogeography of organisms living within the past 50,000 years.  |
Chemical Bonds
How elements interact with one another depends on the number of electrons and how they are arranged. When an atom does not contain equal numbers of protons and electrons it is called an ion. Because the number of electrons does not equal the number of protons, each ion has a net charge. For example, if sodium loses an electron, it now has 11 protons and only 10 electrons, leaving it with an overall charge of +1. Positive ions are formed by losing electrons and are called cations. Negative ions are formed by gaining electrons and are called anions. Elemental anionic names are changed to end in -ide. As an example, when chlorine becomes an ion it is referred to as chloride.
Ionic and covalent bonds are strong bonds formed between two atoms. These bonds hold atoms together in a relatively stable state. Ionic bonds are formed between two oppositely charged ions (an anion and a cation). Because positive and negative charges attract, these ions are held together much like two oppositely charged magnets would stick together. Covalent bonds form when electrons are shared between two atoms. Each atom shares one of their electrons, which then orbits the nuclei of both atoms, holding the two atoms together. Covalent bonds are the strongest and most common form of chemical bond in organisms. Unlike most ionic bonds, covalent bonds do not dissociate in water.
Covalent bonds come in two varieties: polar and non-polar. A non-polar covalent bond occurs when electrons are shared equally between the two atoms. Polar covalent bonds form when the electrons are shared unequally. Why does this occur? Each element has a known electronegativity: a measure of their affinity for electrons. Some elements, such as oxygen, are very electronegative because they strongly attract electrons from other atoms. Hydrogen, meanwhile, has low electronegativity and thus weakly attracts electrons, in comparison. Polar covalent bonds form when the two atoms involved have significantly different electronegativities. In biological systems, this occurs when oxygen bonds with hydrogen and when nitrogen (also quite electronegative) bonds with hydrogen.
When oxygen and hydrogen bond, for example, the shared electrons are pulled more strongly toward oxygen and thus farther away from hydrogen’s nucleus. Because the electrons move farther away from hydrogen, it becomes slightly positively charged (δ+). The oxygen becomes slightly negatively charged as the electrons become closer to it (δ–). If two molecules with polar covalent bonds approach one another, they can interact due to the attraction of opposite electrical charges. For example, the slight positive charge of hydrogen in a water molecule can be attracted to the slight negative charge of oxygen in a different water molecule (Figure 3). This interaction between two polar molecules is called a hydrogen bond. This type of bond is very common in organisms. Notably, hydrogen bonds give water the unique properties that sustain life. If it were not for hydrogen bonding, water would be a gas rather than a liquid at room temperature.

WATER IS CRUCIAL TO MAINTAINING LIFE
Do you ever wonder why scientists spend time looking for water on other planets? It is because water is essential to life; even minute traces of it on another planet can indicate that life could or did exist on that planet. Water is one of the more abundant molecules in living cells and the one most critical to life as we know it. Approximately 60–70 percent of your body is made up of water. Without it, life simply would not exist.
- Water is polar. The hydrogen and oxygen atoms within water molecules form polar covalent bonds. The shared electrons spend more time associated with the oxygen atom than they do with hydrogen atoms. There is no overall charge to a water molecule, but there is a slight positive charge on each hydrogen atom and a slight negative charge on the oxygen atom. Because of these charges, the slightly positive hydrogen atoms repel each other and form the unique shape. Each water molecule attracts other water molecules because of the positive and negative charges in the different parts of the molecule. Water also attracts other polar molecules (such as sugars) that can dissolve in water and are referred to as hydrophilic (“water-loving”).
- Water stabilizes temperature. The hydrogen bonds in water allow it to absorb and release heat energy more slowly than many other substances. Temperature is a measure of the motion (kinetic energy) of molecules. As the motion increases, energy is higher and thus temperature is higher. Water absorbs a great deal of energy before its temperature rises. Increased energy disrupts the hydrogen bonds between water molecules. Because these bonds can be created and disrupted rapidly, water absorbs an increase in energy and temperature changes only minimally. This means that water moderates temperature changes within organisms and in their environments.
- Water is an excellent solvent. Because water is polar, with slight positive and negative charges, ionic compounds and polar molecules can readily dissolve in it. Water is, therefore, what is referred to as a solvent—a substance capable of dissolving another substance. The charged particles will form hydrogen bonds with a surrounding layer of water molecules.
- Water is cohesive. Have you ever filled up a glass of water to the very top and then slowly added a few more drops? Before it overflows, the water actually forms a dome-like shape above the rim of the glass. This water can stay above the glass because of the property of cohesion. In cohesion, water molecules are attracted to each other (because of hydrogen bonding), keeping the molecules together at the liquid-air (gas) interface, although there is no more room in the glass. Cohesion gives rise to surface tension, the capacity of a substance to withstand rupture when placed under tension or stress. When you drop a small scrap of paper onto a droplet of water, the paper floats on top of the water droplet, although the object is denser (heavier) than the water. This occurs because of the surface tension that is created by the water molecules. Cohesion and surface tension keep the water molecules intact and the item floating on the top. It is even possible to “float” a steel needle on top of a glass of water if you place it gently, without breaking the surface tension. These cohesive forces are also related to the water’s property of adhesion, or the attraction between water molecules and other molecules. This is observed when water “climbs” up a straw placed in a glass of water. You will notice that the water appears to be higher on the sides of the straw than in the middle. This is because the water molecules are attracted to the straw and therefore adhere to it. Cohesive and adhesive forces are important for sustaining life. For example, because of these forces, water can flow up from the roots to the tops of plants to feed the plant.
Buffers, pH, Acids, and Bases
The pH of a solution is a measure of its acidity or alkalinity. The pH scale ranges from 0 to 14. A change of one unit on the pH scale represents a change in the concentration of hydrogen ions by a factor of 10, a change in two units represents a change in the concentration of hydrogen ions by a factor of 100. Thus, small changes in pH represent large changes in the concentrations of hydrogen ions. Pure water is neutral. It is neither acidic nor basic and has a pH of 7.0. Anything below 7.0 (ranging from 0.0 to 6.9) is acidic, and anything above 7.0 (from 7.1 to 14.0) is alkaline. The blood in your veins is slightly alkaline (pH = 7.4). The environment in your stomach is highly acidic (pH = 1 to 2). Orange juice is mildly acidic (pH = approximately 3.5), whereas baking soda is basic (pH = 9.0).
Acids are substances that provide hydrogen ions (H+) and lower pH, whereas bases provide hydroxide ions (OH–) and raise pH. The stronger the acid, the more readily it donates H+. For example, hydrochloric acid and lemon juice are very acidic and readily give up H+ when added to water. Conversely, bases are those substances that readily donate OH–. The OH– ions combine with H+ to produce water, which raises a substance’s pH. Sodium hydroxide and many household cleaners are very alkaline and give up OH– rapidly when placed in water, thereby raising the pH.

How is it that we can ingest or inhale acidic or basic substances and not die? Buffers are the key. Buffers readily absorb excess H+ or OH–, keeping the pH of the body carefully maintained in the aforementioned narrow range. Carbon dioxide is part of a prominent buffer system in the human body; it keeps the pH within the proper range. This buffer system involves carbonic acid (H2CO3) and bicarbonate (HCO3–) anion. If too much H+ enters the body, bicarbonate will combine with the H+ to create carbonic acid and limit the decrease in pH. Likewise, if too much OH– is introduced into the system, carbonic acid will combine with it to create bicarbonate and limit the increase in pH. While carbonic acid is an important product in this reaction, its presence is fleeting because the carbonic acid is released from the body as carbon dioxide gas each time we breathe. Without this buffer system, the pH in our bodies would fluctuate too much and we would fail to survive.
Biological Molecules
Besides water, the molecules necessary for life are organic. Organic molecules are those that contain carbon covalently bonded to hydrogen. In addition, they may contain oxygen, nitrogen, phosphorus, sulfur, and additional elements.There are four major classes of organic molecules: carbohydrates, lipids, proteins, and nucleic acids. Each is an important component of the cell and performs a wide array of functions.
It is often said that life is “carbon-based.” This means that carbon atoms, bonded to other carbon atoms or other elements, form the fundamental components of many of the molecules found uniquely in living things. Other elements play important roles in biological molecules, but carbon certainly qualifies as the “foundation” element for molecules in living things. It is the bonding properties of carbon atoms that are responsible for its important role.
Carbon can form four covalent bonds with other atoms or molecules. The simplest organic carbon molecule is methane (CH4), in which four hydrogen atoms bind to a carbon atom (Figure 5). Carbohydrates include what are commonly referred to as simple sugars, like glucose, and complex carbohydrates such as starch. While many types of carbohydrates are used for energy, some are used for structure by most organisms, including plants and animals. For example, cellulose is a complex carbohydrate that adds rigidity and strength to the cell walls of plants. The suffix “-ose” denotes a carbohydrate, but note that not all carbohydrates were given that suffix when names (e.g., starch).

Lipids include a diverse group of compounds that are united by a common feature. Lipids are hydrophobic (“water-fearing”), or insoluble in water, because they are non-polar molecules (molecules that contain non-polar covalent bonds) . Lipids perform many different functions in a cell. Cells store energy for long-term use in the form of lipids called fats. Lipids also provide insulation from the environment for plants and animals. For example, they help keep aquatic birds and mammals dry because of their water-repelling nature. Lipids are also the building blocks of many hormones and are an important constituent of cellular membranes. Lipids include fats, oils, waxes, phospholipids, and steroids.
Proteins are one of the most abundant organic molecules in living systems and have the most diverse range of functions of all macromolecules. They are all polymers of amino acids. The functions of proteins are very diverse because there are 20 different chemically distinct amino acids that form long chains, and the amino acids can be in any order. Proteins can function as enzymes, hormones, contractile fibers, cytoskeleton rods, and much more. Enzymes are vital to life because they act as catalyst in biochemical reactions (like digestion). Each enzyme is specific for the substrate (a reactant that binds to an enzyme) upon which it acts. Enzymes can function to break molecular bonds, to rearrange bonds, or to form new bonds.
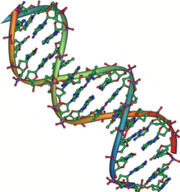
Nucleic acids are very large molecules that are important to the continuity of life. They carry the genetic blueprint of a cell and thus the instructions for its functionality. The two main types of nucleic acids are deoxyribonucleic acid (DNA) and ribonucleic acid (RNA). DNA is the genetic material found in all organisms, ranging from single-celled bacteria to multicellular mammals. The other type of nucleic acid, RNA, is mostly involved in protein synthesis. The DNA molecules never leave the nucleus, but instead use an RNA intermediary to communicate with the rest of the cell. Other types of RNA are also involved in protein synthesis and its regulation. DNA and RNA are made up of small building blocks known as nucleotides. The nucleotides combine with each other to form a polynucleotide: DNA or RNA. Each nucleotide is made up of three components: a nitrogenous base, a pentose (five-carbon) sugar, and a phosphate. DNA has a beautiful double-helical structure (Figure 6).
Additional Resources:
Attribution
Concepts of Biology by OpenStax is licensed under CC BY 4.0. Modified from original by Matthew R. Fisher.

