7 Pharyngeal arches
- Overview of the pharyngeal arches
- Development of external structures
- Fronto-nasal process
- 1st pharyngeal arch
- maxillary process
- 2nd-6th pharyngeal arch
- Pharyngeal grooves
- Pharyngeal pouches
- Development of internal structures
- Pituitary gland
- Salivary glands
- Palate
- Tongue
- Tonsils
- Clinical application of pharyngeal arch development
- Cleft lip/palate
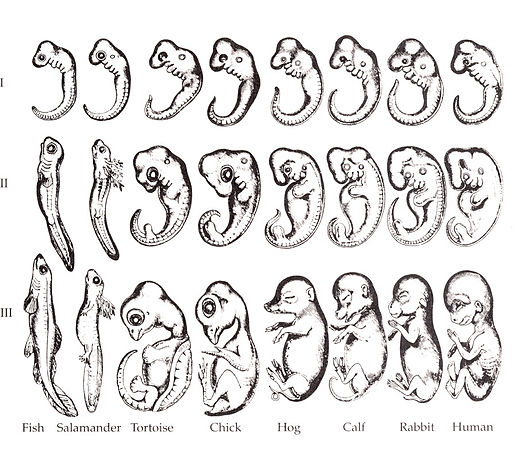
"Embryo drawings", by Ernst Haeckel, is in the Public Domain CC0Overview of the pharyngeal arches
Ontogeny recapitulates phylogeny —Ernst Haeckel
Nothing in biology makes sense except in the light of evolution—Theodosius Dobzhansky
I suppose it is tempting, if the only tool you have is a hammer, to treat everything as if it were a nail— Abraham Harold Maslow
The purpose of this overview section is to conceptually prepare you for a complicated series of steps that go through unexpected transitions. To do so, we discuss the link between evolution and development. If the link seems complicated, you are correct, it is complicated. Evolution explains why transitions happen, your job is to learn what those transitions are.
The human embryo is taking shape. It might seem odd that we look a bit wormy at this early stage, and next we morph into something fishy in appearance. The phrase ontogeny recapitulates phylogeny is hallowed among developmental biologists. It means, more or less, our early embryonic stages look like our evolutionary lineage. That is what the famous drawing (Fig. 7.1) shows: trace our evolutionary lineage (over millions of years), it looks like our embryonic lineage (the 9 months before birth). In this chapter, we see human embryo grow what look like gill arches, then remodel those gill arches into other structures: a mandible and maxilla, ears, and salivary glands. Why not just grow a mandible and ears? Why go through the middle gill arch step? Why do we resemble worms, then fish, and only much later little people? The better question, from the perspective of an evolutionary biologist, is why would we stop developing the way our ancient ancestors did. Would that help us avoid predation, have more offspring or have superior offspring? The answer is apparently no.
That’s the first big concept: evolution is driving, you are a passenger. There may very well be a faster route from there to here, but it won’t help pointing that route out now. The next big concept is we rarely see new structures arise in evolution de novo (from the beginning). Otherwise we'd have wheels, not inefficient legs. Instead, we observe structures changing slightly over time (i.e. we see a lot of different types of legs). This leads to different species having structures with similar shapes but different functions. We call such structures homologues. A bat’s wing, a whale’s flipper, a horse’s leg and your arm have the same basic skeletal pattern: 1 bone, 2 bones, 4 bones (ignore the thumb and big toe, they were added later). All 4 of these limbs are homologous to one another. Their size, shape and purpose are different, but they share the same basic design. It is more efficient to morph a leg into a flipper than it is to design a flipper de novo. Some homologues have very different functions, such as human lungs and fish swim bladders. Conversely, similarity does not mean homology. A fly wing, chicken wing and bat wing, despite their similar function, are not homologous. Similarly, the panda’s (6th) thumb is not a homologue of the human thumb, either. This becomes more apparent when you study the lineage of different species and compare it to their development, a science called evolutionary developmental biology (evo-devo). Can you summarize the difference between our first list of species with homolgous limbs versus the second list of species with non-homologous limbs? In this chapter, when you look at the pharyngeal arches in humans, you might ask why are they numbered 1, 2, 3, 4… 6? Who decided 6 comes after 4? The answer lies in observing homology across species.
Homologues also exist within a single organism. When we discuss homology between two human structures, instead of evolutionary lineage, we are discussing developmental lineage. That is why your arm and leg share the same skeletal pattern, they are homologues. That is also why there is so much similarity between the skin and oral mucosa. Evolution by gene duplication involves fewer steps than generating DNA instruction de novo. Small changes in duplicate DNA can lead to big changes in morphology. Therefore, it is both faster and easier to tweak a working design than create a whole new design de-novo. Human development is rife with examples of recapitulation, where a basic process is re-used with small changes.
Keep in mind humans did not evolve from modern-day worms or fish. Evolution is not a ladder humans have ascended. Modern day fish are as highly evolved as humans are. But the most recent relative a trout and humans share resembled a fish more than a human. The most recent relative a trout and a tapeworm share resembled a worm more than a fish. Now reverse that: our embryonic stages initially look strikingly like some sort of parasitic larva, then more fishy, then kind of lizardy, and finally mammally. Another way to say that is ontogeny recapitulates phylogeny.

"Gill arches supporting the gills in a pike" by Uwe Gille is licensed under CC BY 3.0Now is a good time to say this again: evolution explains why strange transitions happen. Your job is to learn what the transitions are. Before we finish this overview, we would like to point out one more level of complexity. In this chapter you must differentiate between structures with similar names, including the pre-maxillary segment, inter-maxillary segment and maxillary processes, pharyngeal arches, pharyngeal grooves and pharyngeal pouches, and more. Furthermore, most of these structures have multiple names, such as pharyngeal arches, gill arches and branchial arches, which can make comparing this text to others tricky.
If you feel like you need more detail or a different description, here are good resources on embryology and evolution:
- The
Embryology Educationpage- by Dr. Mark Hill at the University of New South Wales, Sydney, Australia
Understanding Evolution- The University of California Museum of Paleontology, Berkeley
Development of the external structures

"Illustration of the seven facial prominences that give rise to specific regions of the face", by Kristina Aldridge, is licensed CC BY 4.0At 4 weeks, the face begins to develop. It is composed of several parts (or prominences), listed in Table 7.1. To use the word prominence means these are not necessarily the same type of thing, but a group of things that scientists can point to and give them names. The prominences we focus on are homologues of gill arches (gill arches are in Fig. 7.2, their human homologues are in Fig. 7.3 upper left). They are quickly remodeled to look more like human parts than gill arches (Fig 7.3, lower right). These are the pharyngeal arches (or branchial arches, although technically the name branchial arches should only be used for vertebrates with gills). Pharyngeal arches are paired segmental bulges on the lateral borders of the primitive pharynx. The two halves of each arch grow medially across the face and neck (the ventral side) and fuse to form an arch. It is worth noting that pharyngeal arches may look like somites, but pharyngeal arches make face and neck structures with plenty of help from neural crest cells, while somites create trunk structures (the pectoral girdle is a mixture of the two). In addition to the arches, one pair of processes (not arches) run into a bump called the inter-maxillary segment, and fuse with it instead.
Is that clear? No? We go into more detail below, but for now recognize you may need to refer to some of this confusing terminology as we continue forward.
| The facial prominences |
|---|
| 2 halves of the Mandibular (1st) arch |
| 2 Maxillary processes |
| Fronto-nasal process |
In lampreys, seven arches become seven pairs of gill supports on the sides of the head. In bony fish, one arch is remodeled into a mandible and middle ear structures, while five become gill supports (only six form in the first place). Humans remodel the first arch the same way as bony fish, plus we remodel our other four (we have no gill supports). If you’ve looked at a lamprey recently, you might have this question: if humans remodel the first arch to produce jaws and teeth, but lamprey do not, what are lamprey teeth? They aren’t enamel or dentin!
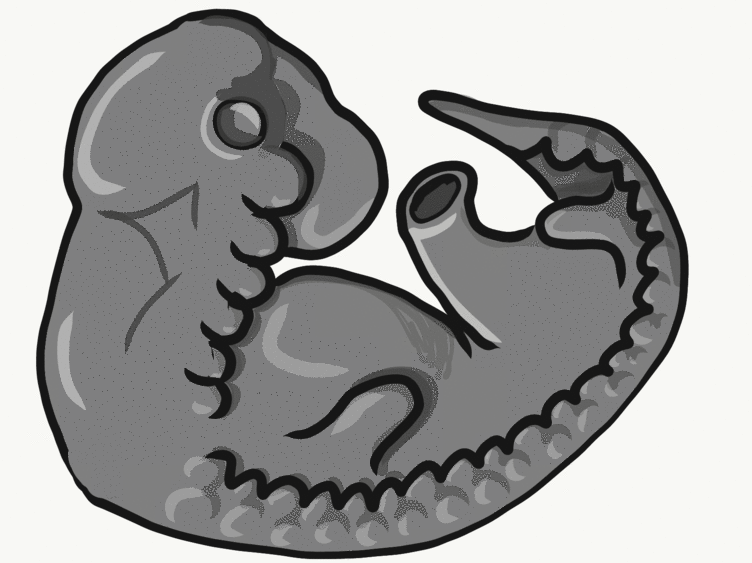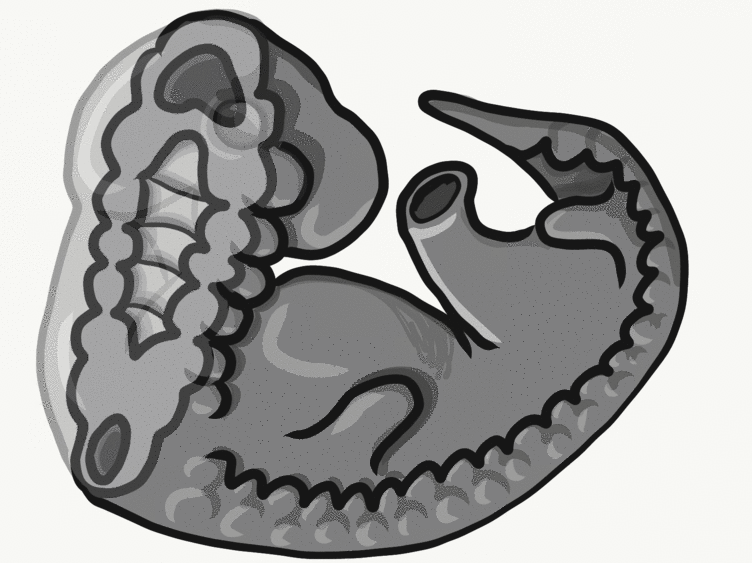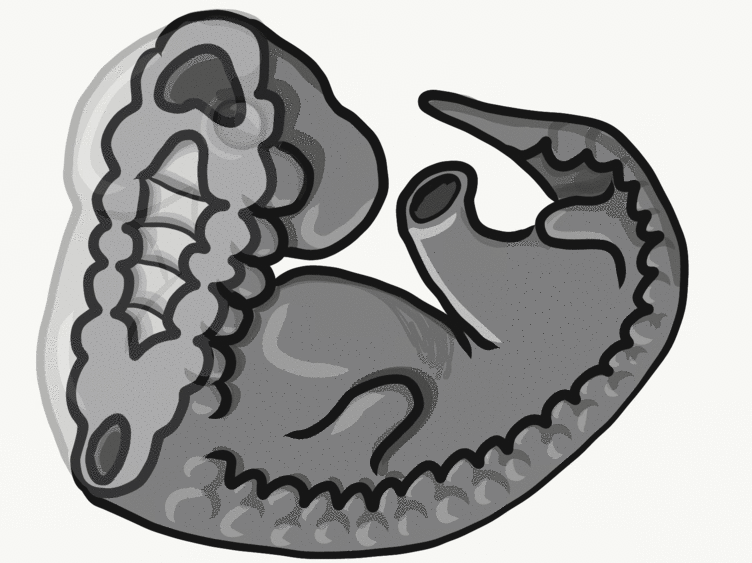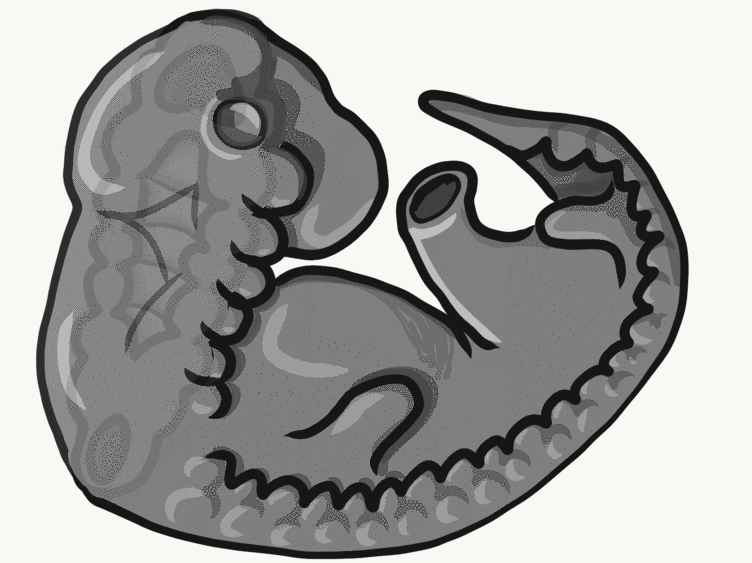
Formation of the pharyngeal arches
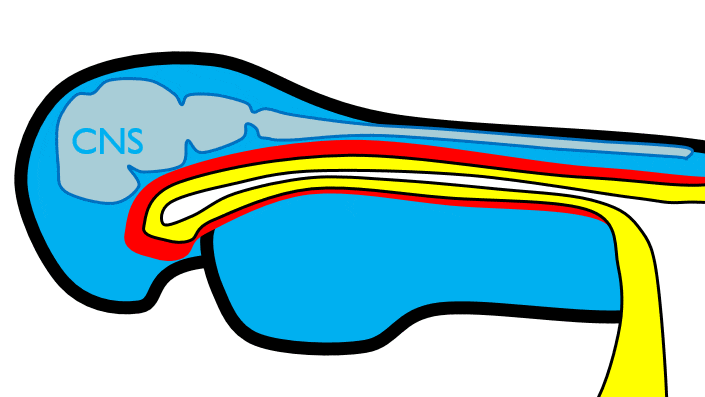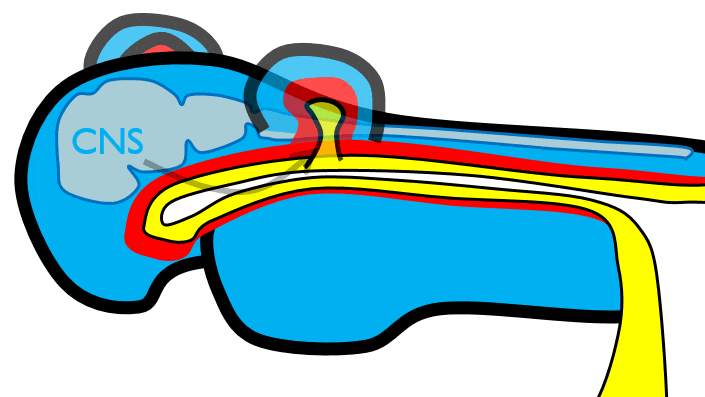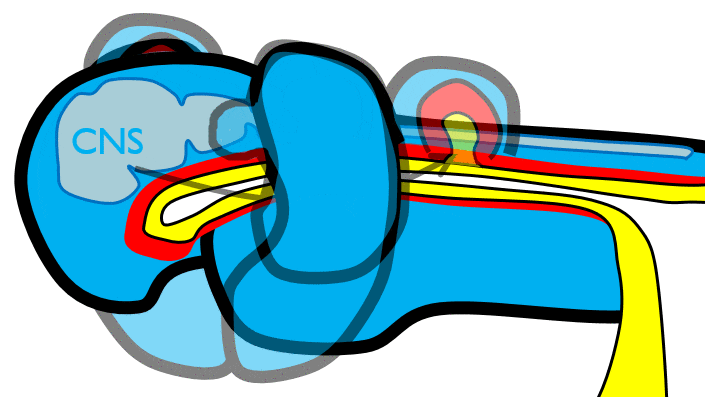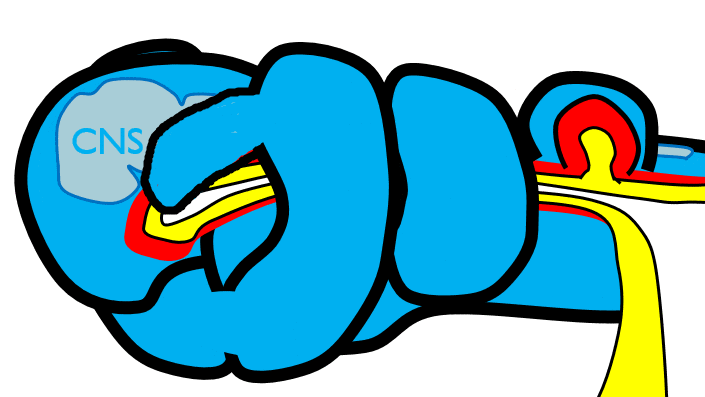
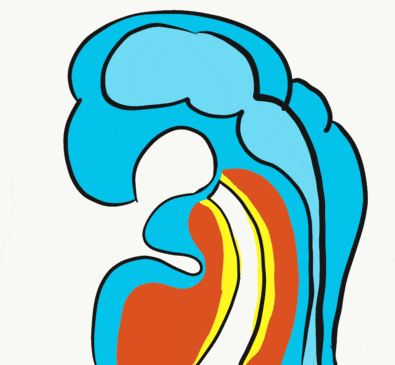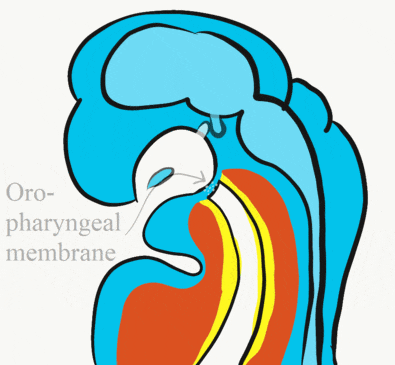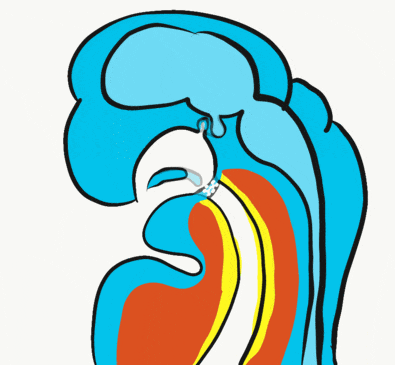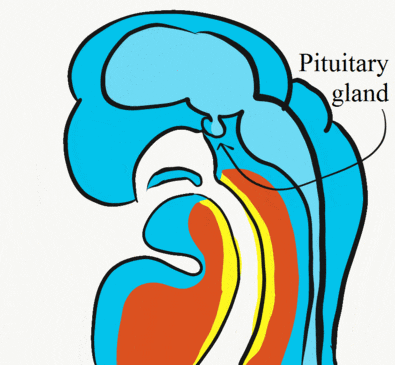
At the 4th week, the primitive foregut has not fused with ectoderm, so no oral or nasal cavities exist. There is visible as a depression in the ectoderm known as the stomodeum (the primitive mouth, Fig. 7.9). Think of this as a bowl-shaped invagination. A small part of that depression, the oro-pharyngeal membrane (or bucco-pharyngeal membrane), separates the stomodeum from the anterior end of the primitive foregut (which invaginated during gastrulation←). The primitive foregut is lined by endoderm, and the stomodeum is ectoderm. Almost everywhere, there is mesoderm between endoderm and ectoderm. However the oro-pharyngeal membrane contains only endoderm and ectoderm. Some amphibians use the oro-pharyngeal membrane to breathe underwater. It is thin enough to allow gas exchange to occur, but prevents the lungs from filling up with water. Humans develop a stomodeum, then remove it.
The pharyngeal arches form under the instruction of homeobox genes←. Activation of homeobox genes (in an anterior-to-posterior pattern) induces the transcription of a program of other genes. These other genes include morphogens← which induce growth of all 3 embryonic germ layers. This causes outward budding on the lateral borders of the pharynx. 5 arches form, starting with arch number 1 (the most anterior arch) and ending with arch number 6 (the most posterior arch). Each arch grows medially to fuse with its partner. Imagine drawing the letter U using two pens. Starting from each corner, bring both pens downwards and meet in the middle. You have drawn an arch. Now, instead of drawing a U on paper, draw on your head. Place both pen tips below your ears and draw across your face, meeting at your mandibular symphysis. You have drawn arch number 1. Now draw 4 more, each U below the next. There, you have 5 arches.
We now have 5 mounds on the outside of the embryo. The valleys between the mounds are called pharyngeal grooves (Fig. 7.6). At t same time as mounds are forming on the outside of the embryo, things are happening inside as well. Within the primitive pharynx, localized growths form invaginations known as the pharyngeal pouches (Fig. 7.7). If you remember GAP, this mnemonic may help you to remember the names of these structures from external to internal: Groove, Arch, Pouch. Or, if you prefer the name pharyngeal cleft rather than groove, the mnemonic becomes CAP. These structures appear one pair at a time, from anterior to posterior, and their fate is listed in Table 7.2. Because the arches are quickly remodeled into other structures, we say that they are transient structures. Their brief existence explains how and why the adult structures in Table 7.2 have the morphology that they do. Recall that in embryology, anterior-to-posterior means head-to-toe (rostral-to-caudal), not ventral-to-dorsal.
| Arch # | Name | Ectoderm and neuro-ectoderm fate | Groove fate |
Mesoderm and neuro-mesenchyme fate | Endoderm (pouch) fate |
|---|---|---|---|---|---|
| 1st | Mandibular arch | Maxillary process –> upper lip epidermis | n/a
(this is not an arch) |
Dermis, maxilla, zygomatic, palatine, vomer | n/a
(this is not an arch) |
| Lower lip epidermis, Trigeminal nerve (CNV). | External acoustic meatus | Dermis, mandible, malleus, incus | Eustachian tube | ||
| 2nd | Hyoid arch | Epidermis, Facial nerve (CNVII) | disappears | Dermis, most of the hyoid bone, stapes | Palatine tonsils |
| 3rd | Epidermis, Glossopharyngeal nerve (CNIX) | Dermis, the rest of the hyoid bone | Thymus, Parathyroid glands | ||
| 4th | Epidermis, Vagus nerve (CNX) | Dermis, Thyroid cartilage, epiglottis | Parathyroid, Thyroid glands | ||
| 5th never forms | |||||
| 6th | Epidermis, Vagus nerve (CNX) | Dermis, the other laryngeal cartilages | Larynx tissues | ||
The 1st pharyngeal arch
During neurulation←, neural crest cells← undergo an epithelial-to-mesenchymal transition←, migrate away from the neural tube and into the mesoderm of the pharyngeal arches. Once there, neural crest cells differentiate← into neuro-mesenchymal stem cells (NMSCs). These cells guide remodeling of the pharyngeal arches. The first step is to begin forming an upper jaw off the lower jaw. The neuro-mesenchymal stem cells release morphogens← which induce localized proliferation within the mandibular arch (the 1st pharyngeal arch), forming the maxillary processes (Fig.7.5). The rest of the mandibular arch grows medially to form the lower jaw. The maxillary processes also grow medially to form the upper jaw. You might expect there to be a mouth between the upper and lower jaw. Not yet, between them is stomodeum, which at this time is still not connected to the primitive foregut.
Tissue also grows on the medial and lateral side of each nasal placode (Fig. 7.6). The two medial nasal processes fuse to form the inter-maxillary segment (or globular process). Because some teeth develop from the inter-maxillary segment, we will discuss it more. The two lateral nasal processes develop into the alae of the nose. This is the last mention of the lateral nasal processes.

Fusion of the 1st pharyngeal arch
The mandibular arch forms on the lateral edges of the embryo during the 4th week of development and grows medially. The two halves fuse by the end of the 4th week of development, creating a single structure that becomes the mandible, plus some nearby tissue. For the two halves of the mandibular arch to grow medially, mesenchyme← is removed. This requires expression of the enzyme hyaluronidase, which digests hyaluronic acid← found in ground substance←. This allows epithelial cells to fuse with epithelial cells from the other half of the arch. Fusion requires matching CAMs← and desmosomes←. The mesoderm of one arch also fuses with mesoderm of its partner, which requires matching the correct integrin← to fibronectin←.
Later, neuro-mesenchyme of the mandibular arch differentiates← into a cartilaginous structure known as Meckel’s cartilage. Parts of Meckel’s cartilage undergo endochondral ossification to become part of the mandible and middle ear bones, the rest undergoes apoptosis←.
Fusion of the maxillary processes and inter-maxillary segment
The pair of maxillary processes grow medially in the 4th week, but run into the inter-maxillary segment and fuse with it by the 10th week of development (Fig. 7.6). The upper lip, therefore, is formed of three parts: the left maxillary processes, the right maxillary process, and the inter-maxillary segment (the lower lip is formed from two parts: the left and right halves of the mandibular arch). The philtrum is the middle section derived from the inter-maxillary segment. It does not serve a function in humans, it happens to be there because of how we develop (like the choice of wood used in the record player shelf mnetioned in the preface). Anatomy textbooks typically describe functions of organs based on their adult form (for example, read chapter 1 of the Openstax Anatomy and Physiology textbook). Some have tried to describe the function of the human philtrum based on its shape and location. If you study development (ontogeny) and evolution (phylogeny), anatomy can make more sense.

Fate of the pharyngeal grooves and pharyngeal pouches
If you were a fish, most of the pharyngeal grooves would develop into gills. As creatures of the land, grooves are either filled in or develop into other useful structures. Between the first and second arches, the first pharyngeal groove invaginates further and forms a tube that becomes the external acoustic meatus. It is lined by ectoderm. The other pharyngeal grooves disappear. One the opposite side, within the primitive pharynx, the pharyngeal pouches invaginate and grow towards the grooves. The first pharyngeal pouch elongates into a tube that is fated to become the Eustachian tube, connecting the pharynx to the middle ear (the internal acoustic meatus forms when bone tissue grows around cranial nerve VIII, connecting the inner ear to the brain). The Eustachian tube is lined by endoderm. The other pharyngeal pouches invaginate and form tonsillar and glandular tissue. Tonsils are covered by an endoderm-derived epithelium, but the white blood cells of the germinal centers migrate there from bone marrow (which, like most connective tissues, is derived from mesoderm). The mesoderm and neuro-mesenchyme between the first pharyngeal groove and pharyngeal pouch form middle ear structures, including the malleus, incus and stapes bones.
Development of the palate and other internal structures

"Embryonic development of murine SMG and SL glands." by Cristina Porcheri and Thimios A. Mitsiadis is licensed under CC BY-SA 4.0Development of the salivary glands
The salivary glands develop in a process that begins similarly to neurulation←. Placodes form on the ectoderm starting between weeks 4 through 12. Notice this means salivary glands develop from the outside of the embryo, not from pharyngeal pouches. Growth of placodes is under the control of morphogens← including members of the FGF family. Salivary gland placodes grow and invaginate from there. Eventually, stem cells← differentiate← into a number of epithelial cell types. The ducts are mostly simple cuboidal epithelia←. These epithelial cells have different functions based on their distal-to-proximal location along the duct. The differentiation of salivary gland cells along a proximal-to-distal axis is guided by planar cell polarity← morphogens, including members of the Wnt family. Some cells in the acini differentiate into myo-epithelial cells. Myo-epithelial cells are ectodermal by lineage, and therefore epithelial, despite looking and acting like smooth muscle cells. Myo-epithelial cells express genes mostly used by muscle cells. For an epithelial cell to share this morphology it must reverse earlier decisions it made. Histone packing and DNA methylation is removed from genes shut down as ectodermal cells initially adopted an epithelial fate.
Formation of the pituitary and mouth
Inside the stomodeum, a single invagination of ectoderm forms, along the medial portion of the roof (so far, processes and pouches have been left/right pairs). This invagination is named Rathke's pouch. It grows and meets a downward budding of neuro-ectoderm. These two fuse to form the pituitary gland. The ectoderm forms the glandular half (adenohypophysis), and the neuro-ectoderm forms the infundibulum and neural half (neurohypophysis) of the pituitary gland. Rathke’s pouch fills in as the two halves of the pituitary fuse, but it is possible a small depression will remain.
At the same time, the oro-pharyngeal membrane undergoes apoptosis←. This connects the primitive foregut and stomodeum, forming the primitive oro-nasal cavity. Finally, the mouth and anus are connected! The oral mucosa therefore develops from ectoderm of the stomodeum. The lining of the pharynx is derived from the endoderm of the primitive foregut. The lining of the tongue is a mashup of the two.
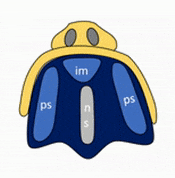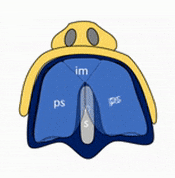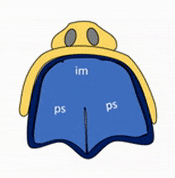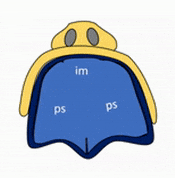
Formation of the palate
Shortly after the lips begin forming, the palate begins to form as well, dividing the primitive oro-nasal cavity into a more mature oral cavity and nasal cavities. The palate has 3 parts that fuse with each other, and with the nasal septum. The primary palate grows from the inter-maxillary segment, and two palatal shelves (or secondary palate) grow from the maxillary processes.
| Structure | Lineage | Forms during | Fuses with |
|---|---|---|---|
| Primary palate (pre-maxillary segment) |
inter-maxillary segment (globular process) |
6th week | Secondary palate: 9th week |
| Secondary palate
(Palatal shelves) |
Maxillary process | 7th week | The other palatal shelf: 9th week |
| Primary palate: 9th week | |||
| Nasal septum: 12th week |

The first part of the palate to form is the primary palate, which develops from the inter-maxillary segment. When it forms, it partially divides the future oral and nasal cavities (Fig. 7.9). Next, two palatal shelves grow off of the maxillary processes (Fig. 7.10 and 7.11). The palatal shelves first grow inferiorly, then change direction and grow medially. At this time, the developing tongue must move out of the way. This allows the palatal shelves to meet and fuse with the primary palate, as well as each other (forming the secondary palate). The fusion happens in an anterior-to-posterior direction. All of this growth is directed by morphogens, including FGFs and BMPs.
Maxillary incisors develop from the primary palate, while maxillary canines, pre-molars and molars develop from the secondary palate. At the 3-way corner where the primary palate and the two palatal shelves fuse, a small hole remains, the incisive foramen. The incisive foramen houses the nasopalatine artery and vein and a branch of the trigeminal nerve. The oral mucosa above this foramen has a bump named the incisive papilla, which shares more in common with olfactory epithelium than it does oral epithelium (it is the homologue of the vomeronasal organ found in many vertebrates). Where the two palatal shelves fuse leaves a ridge on the overlying oral mucosa called the (median) palatine raphe.
Keep in mind that we are referring to the entire palate. Much later, anterior portions of palate mesoderm undergo endochondral ossification and form the palatine bones and the palatine processes of the maxilla (the hard palate). The rest of palatal mesoderm differentiates into muscle tissue, forming the soft palate. Time out for spelling: this is the palate, not an artist’s palette of colors, nor a pallet used in shipping, not even a plate on which we place a tasty dinner. Therefore, foodstuffs shipped on a pallet, cooked by a chef with a harmonious palette, served to us on a plate, will be enjoyed for their flavor when they hit our palate because we have a refined palate (an appreciation for flavor). Got it? English is fun.
The nasal septum grows inferiorly at this time. It fuses with the completed palate around the 12th week of development. This creates paired nasal cavities. Initially, mesoderm differentiates← into the ethmovomerine cartilage, and then partially undergoes endochondral ossification to generate a bony portion (parts of the ethmoid and vomer) and leaving a cartilaginous portion. Ossification begins from a lateral pair of ossification centers, therefore the early septal bones develop as two layers (lamella) which fuse to form a single bony septum. The two layers are not the ethmoid and vomer (top-to-bottom) portions, but left and right. Why does a single septum develop from a left and right half? The same reason as the mandible: they are induced by neural crest cells←, which arise as distinct groups of cells on the left and right side of the neural tube. Taking another look at the illustrations of neurulation← may help.

Development of the tongue
The tongue is a hybrid structure. It forms from multiple parts making its development complicated. Tongue development begins during the 4th week, after the pharyngeal arches fuse along the bottom of the primitive foregut and future oral cavity. The tongue develops from first four pharyngeal arches (although the contribution of the 2nd arch mostly disappears). Formation of the tongue involves proliferation and fusion, followed by apoptosis← to give the tongue mobility. The tongue is connected to four cranial nerves. That seems like a lot of nerves, doe it really need that many? The innervation of the tongue is easily explained by its development: four arches correspond to four cranial nerve connections. The oral mucosa, sub-mucosa and musculature of the tongue are more complicated.
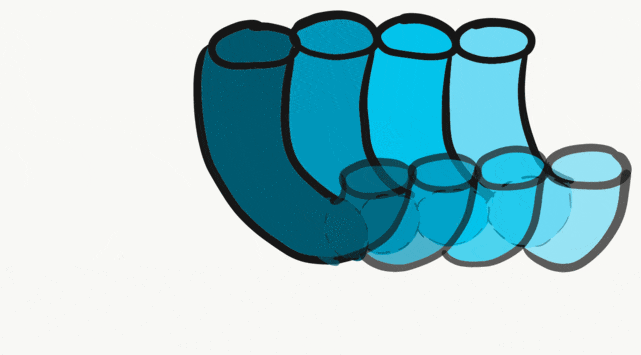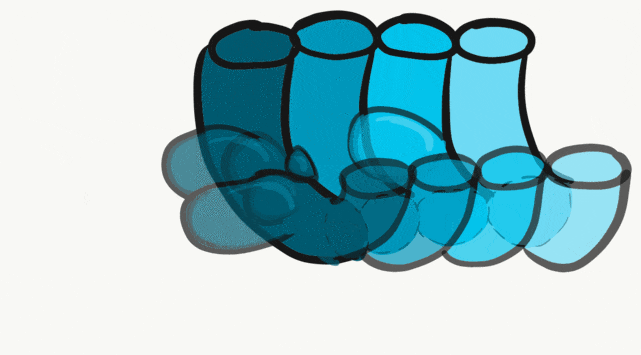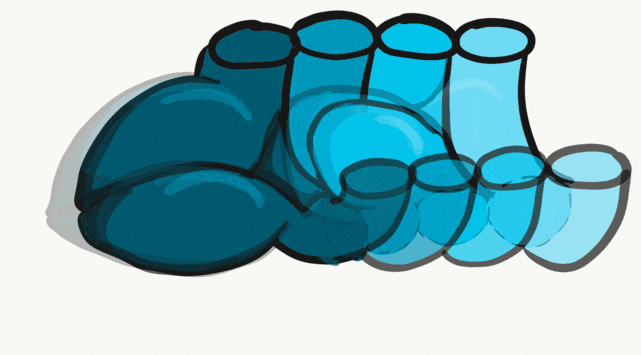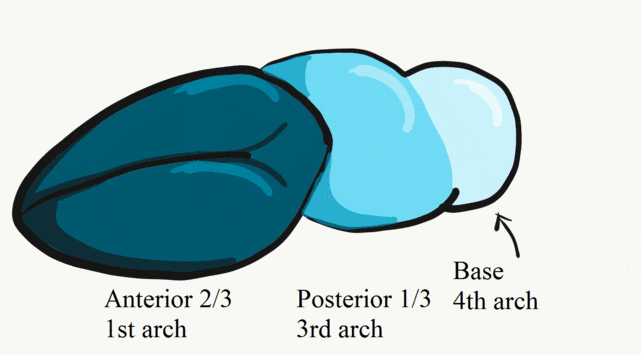
During the 4th week, the left half of each pharyngeal arch fuses with the right half along the floor of the future oral cavity and pharynx. A single triangular-shaped tuberculum impar proliferates off the first pharyngeal arch, followed by two lateral lingual swellings. Because they come from the first pharyngeal arch, their lining is not endoderm like the other arches, but ectoderm from the stomodeum. As these swellings grow, the 3rd and 4th arch develop a swelling named the copula, which grows over the 2nd arch. Fusion of these structures occurs during the 8th week. The median lingual sulcus forms where the left and right lateral lingual swellings fuse. The sulcus terminalis forms where the 1st and 3rd pharyngeal arches fuse. This border between the anterior and posterior portion of the tongue is obvious due to the difference in lineage on either side.
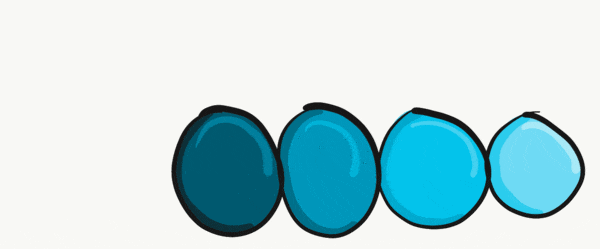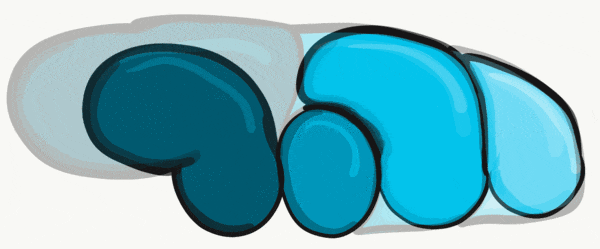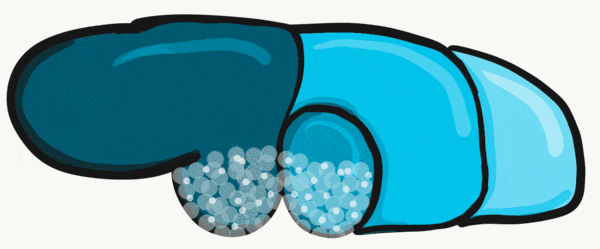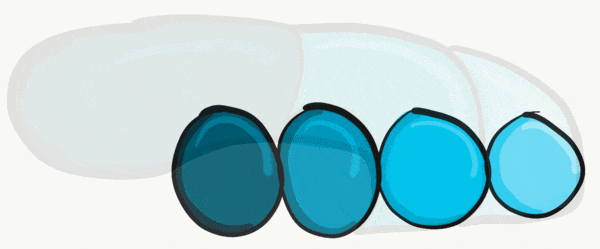
Apoptosis← of tongue tissue on the ventral side leaves the tongue attached at the base, and freer to move around . Apoptosis does not remove all the tissue on the anterior portion. A small amount of mucous membrane remains, named the lingual frenulum. An invagination forms posterior to the sulcus terminalis and grows deeper, forming the thyroid gland. This process is similar to the way the anterior pituitary or the neural tube← form. It leaves behind a small depression named foramen cecum, which is a confusing name because foramen means hole, but this foramen fills in most of the way, making it more of a pouch. Similar to Rathke's pouch, it serves no purpose in humans, it’s a remnant of epithelial tissue proliferation.
The oral mucosa of the tongue is complicated. The outer surface is a stratified squamous epithelium← with two separate lineages. Because the anterior 2/3rds of the dorsal surface of the tongue develops from the mandibular arch, it shares lineage with the surface of the stomodeum, which is ectodermal. The ventral surface of the tongue also develops from the mandibular arch. However, the ectoderm undergoes apoptosis, allowing endoderm from the primitive foregut to cover the ventral surface. As a result, the epithelium of the anterior 2/3rds of the dorsal surface is thicker, and more closely resembles the rest of the oral mucosa. The ventral surface has a thinner epithelial lining, and more closely resembles the lining of the pharynx. The dorsal surface of the posterior 1/3rd of the tongue, coming from the 3rd and 4th arch, is also endodermal.
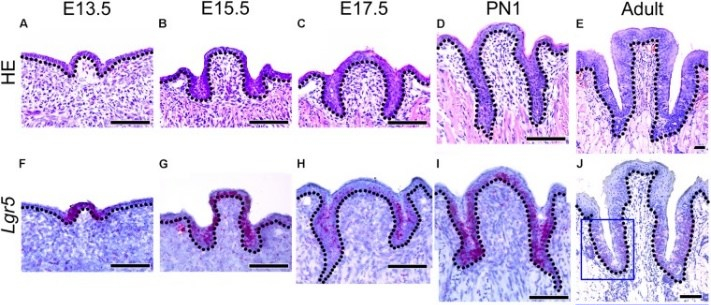
Morphology of developing CVP and expression patterns of Lgr5 and FGF10 during CVP development"” by Sushan Zhang et al is licensed under CC BY 4.0 / croppedDevelopment of the lingual papillae
Like the oral mucosa of the tongue, the lineage of the lingual papillae is either ectoderm or endoderm. The filiform and fungiform papillae develop from invaginations of the ectoderm, while the foliate and circumvallate from invaginations of endoderm. They form by a process similar to neurulation←. The growth and differentiation← of the papillae is guided by morphogens← secreted by underlying neuro-mesenchyme, including members of the FGF and Wnt families. Keratinocytes develop from an ectodermal precursor, while taste buds (including those in the soft palate and pharynx) are induced to develop from ectodermal or endoderm precursors starting the 8th week of development. Older evidence suggests taste bud differentiation depends on neural connections, but newer evidence suggests taste buds develop in response to the Sonic Hedgehog morphogen. By adulthood, both keratinocytes and taste bud cells continue to develop from a shared epithelial stem cell←, and both are replenished throughout life. Whether the lineage of this stem cell is endodermal, ectodermal or both is not known.
The connective tissue (lamina propria, sub-mucosa, and vasculature) of the tongue is derived from neuro-mesenchyme. The skeletal muscle tissue is derived from somite mesoderm, guided by morphogens← secreted from the neuro-mesenchyme.
Clinical applications of pharyngeal arch development
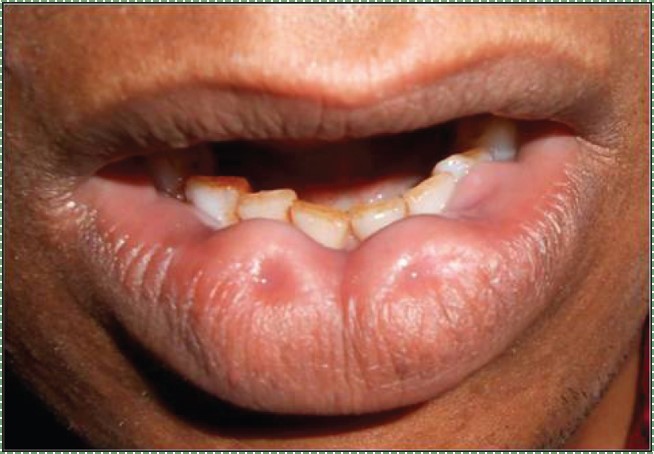
Bilateral congenital lower lip ” by Vela Desai is licensed CC BY-SA 4.0Lip pits
Incomplete fusion of the pharyngeal arches leads to a number of conditions, some more severe than others. Two benign conditions include a lower labial pit, which forms when the two halves of the mandibular arch fail to fuse completely.

"Commissural Pit" by the National Human Genome Research Institute is in the Public Domain, CC0Commissural lip pits (or congenital lip pits) may form between the maxillary processes and mandibular arch. These are examples of cosmetic variations rather than congenital malformations.
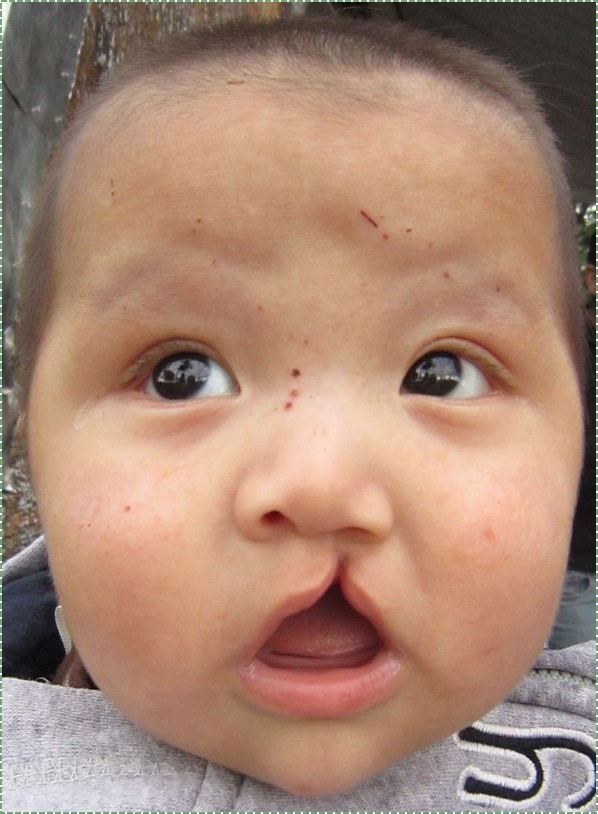
own work” by James Heilman, MD is licensed CC BY-SA 4.0Cleft lip
Incomplete fusion of either maxillary process with the inter-maxillary segment leads to the formation of a cleft lip. This can occur either on the left, right (unilateral) or both (bilateral) borders of the philtrum, although a left unilateral cleft lip is the most common. Cleft lips are more common and more severe in male children. A cleft lip may be accompanied by a cleft palate.
Cleft lip and palate occur in about 1 in 1000 births, making them a relatively common congenital malformation. Risk factors include older mothers, mothers who smoke during pregnancy or who take certain medications (e.g. some anti-convulsants). There are many genetic risk factors for cleft lip and palate, some examples are listed in Table 7.4.
| Gene name | Class of gene | Function |
|---|---|---|
IRF6 |
Transcription factor | Induced during development of mesoderm. |
MSX1 |
Homeobox transcription factor← | Limb patterning |
BMP4 |
Morphogen← | Induction and patterning of bone tissue, teeth and limbs |
FGF10 |
Morphogen | Induction and patterning of connective tissue |
Hyal2 |
Digestive enzyme | Digests hyaluronic acid prior to fusion of the lip or palate |
p63 |
Transcription factor | Controls desmosome protein expression during fusion of the lip or palate |
Epithelial Cadherin 1 |
Cell adhesion molecule← | Allows epithelial cells to connect during fusion of the lip or palate |
A cleft lip can cause difficulty with nursing, as it hinders the formation of a good seal around a nipple. With proper instruction, babies with cleft lip can be breast-fed or bottle-fed using a regular bottle. A cleft lip may cause problems with learning speech. Learning to speak requires sound mimicry, and because a cleft lip alters vocal sounds, it interferes with successful mimicry. Speech and hearing therapy help alleviate these problems. An increased risk of oro-nasal infections is also a concern. The preferred treatment for cleft lip is to seal the gap with surgery at 10 weeks of age. Surgery can leave behind a scar, but otherwise is highly successful.

A 16 year old girl with unilateral complete cleft palate" by Ghulam Fayyaz is licensed CC BY-SA 4.0Cleft palate
Incomplete fusion of the primary palate and/or the palatal shelves leads to a cleft palate. A cleft palate may or may not be accompanied by a cleft lip. Cleft palate is more common in females.
Cleft palate causes difficulty with nursing, because a child cannot create suction with an opening from the oral cavity into the nasal cavity. There are a number of specialty bottles that help babies with cleft palate bottle feed. Similar to cleft lip, a cleft palate can lead to difficulty learning speech. Disruption of palate formation may also lead to shape changes in the Eustachian tubes. The Eustachian tubes develop from the first pharyngeal pouches— close to where the palatal shelves bulge off the maxillary processes. Changes in the shape of the Eustachian tube alter its ability to regulate middle ear pressure, which leads to an increased risk of hearing loss.

Orofacial clefts are categorized first as being a cleft lip (CL), a cleft palate (CP), or a cleft lip and palate (CL/P). A unilateral cleft lip or palate affects just the left or right side, while a bilateral cleft affects both sides. An incomplete cleft palate involves incomplete fusion between the primary palate and a palatal shelf, while a complete cleft also involves incomplete fusion between the two palatal shelves.

Pretreatment extraoral photos of bilateral cleft lip and palate individual“ by Lourdes Martínez Motta and Jessica Sánchez Huanca is licensed under CC BY-NC-SA 4.0The preferred treatments for cleft palate include Naso-Alveolar Molding (NAM), followed by several surgeries. NAM involves screwing or taping an appliance to the maxilla at around 10 months of age. The appliance slowly pulls the regions of the upper lip derived from the maxillary processes in an anterio-medial direction, towards the inter-maxillary segment. Using such an appliance reduces the amount of surgery required to correct the cleft, relying more on guided growth of the child’s tissues. The appliance is adjusted by an orthodontist every two weeks for about a year. This can get tissues closer together, but they won’t fuse. Multiple surgeries follow in the treatment of cleft palate– it is a complicated region, made all the more complex by the fact the child is growing fast. It is not ideal to wait for a child to stop growing for the same reason early intervention for cleft lip was important: orofacial clefts hinder speech development.
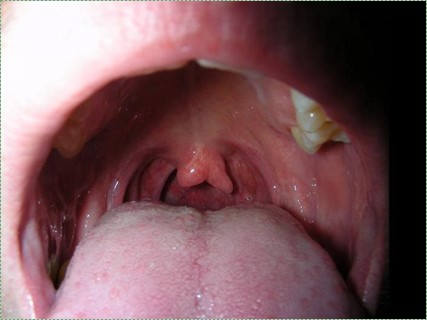
Own work" by Adam6611 is in the Public Domain CC0Cleft uvula
A cleft uvula is the least complicated form of cleft palate, and should be considered a cosmetic variation rather than a congenital malformation. A cleft uvula still closes off the nasopharynx during swallowing.
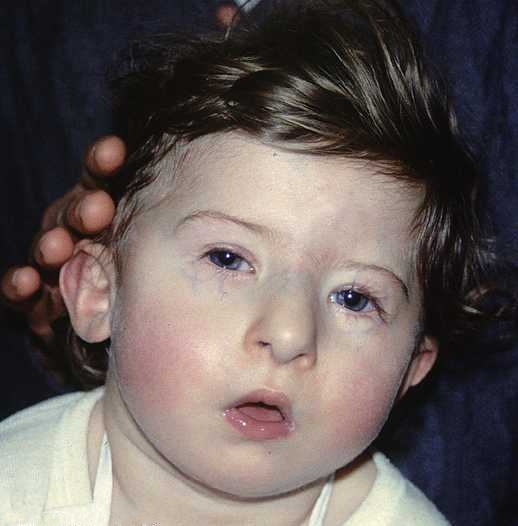
DiGeorge syndrome1" by Prof Victor Grech is licensed under CC BY-SA 3.0DiGeorge syndrome
DiGeorge syndrome (or 22q11.2 deletion syndrome) is caused by a spontaneous (not inherited) deletion to a part of chromosome 22. The deletion removes many genes. One lost gene is TBX1, a transcription factor that activates FGF in the pharyngeal arches. Without FGF, neural crest cells that migrate to the pharyngeal arches die after arrival. This leads to a wide variety of craniofacial abnormalities, including cleft lip/palate, multiple distubances in tooth development (covered in chapters 8, 9 and 10), immune system dysfunction caused by malfomation of the thymus from pharyngeal pouch 3, and dangerous defects to the aorta (whos development is also guided by neural crest cells). Despite severly impacting both the immune system and cardiovascular system, patients with DiGeorge syndrome can have a normal life expectancy with proper and timely surgical interventions.
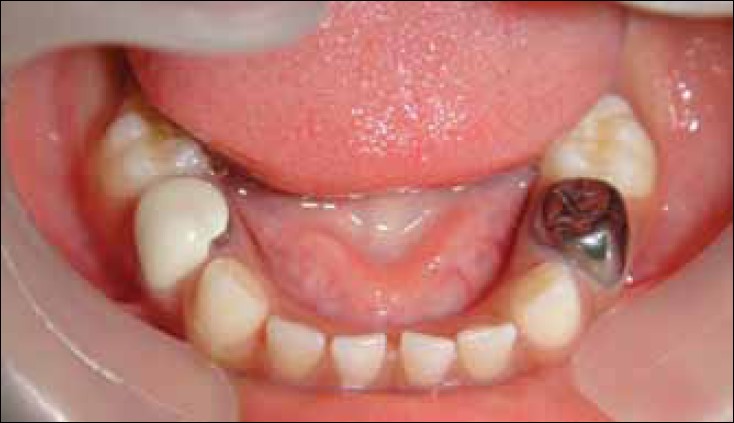
Stainless steel and preveneered crowns after cementation“ by Waleed M Bin AlShaibah, et al is licensed under CC BY-NC-SA 4.0Becasue of the many roles neural crest cells play in formation of the pharyngeal arches and teeth, management of dental issues in pateints with DiGeorge syndrome can be quite complicated. In the next three chapters, think about why loss of neural crest cells inhibits the formation of dentin and enamel. For now, it is enough to know that patients with DiGeorge syndrome often benefit from crowns. The preferred material in pediatrics and for patients with mental and physical disabilities like DiGeorge syndrome are Preformed Metal Crowns (PMCs, stainless steel crowns). PMCs are more durable than (white) composites or amalgams, making them optimal for patients who have difficulty controlling muscles to limit occlusal forces (pdf download on the history of PMCs).
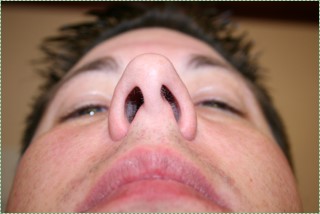
Nostrils before" by Jeff and Mandy G is licensed CC BY SA 2.0Deviated nasal septum
If the nasal septum grows at an angle as it is developing, it leads to a deviated septum. In fact, it is rare for the septum to develop in a symmetrical fashion. 80% of people have some nasal septum deviation, usually without symptoms. Complications can arise because of nasal cavity physiology. The paired nasal cavities contain erectile tissue (areolar connective tissue←) below the nasal mucosa. This tissue undergoes a nasal cycle, alternating between one side swelling shut and the other remaining open for breathing. This prevents the nasal cavities from drying out from constant use. But for someone with a significant nasal septum deviation, it leads to difficulty breathing when the larger cavity swells shut. A relatively simple surgery called septoplasty can be done to increase the size of the smaller nasal cavity. Septoplasty is not the same as the plastic surgery procedure rhinoplasty, where the shape of the nose is altered.

Photo" by Kozlovsk is licensed CC BY SA 3.0Palatal torus
Excessive growth of the palatal shelves can create a palatal torus (or torus palatinus), another example of a cosmetic variation. A palatal torus requires no treatment, as it usually does not cause any health-related issues, aside from complicating the fit to dentures. About 20-30% of the population has some degree of palatal torus. A mandibular torus, on the other hand can develop later in life, often as a response of bone tissue to bruxism (nurture). However, because mandibular tori develop more frequently in Asian and Inuit populations, this suggests there may be genes involved as well (nature).

Ankyloglossia
Ankyloglossia (tongue tie) is the persistence of tissue anchoring the tongue to the floor of the mouth. Most of the ventral side of the mandibular arch should undergo apoptosis←, leaving behind a small lingual frenulum. However, with inadequate apoptosis, a pronounced lingual frenulum results, limiting the mobility of the tongue. This causes problems with breastfeeding and learning speech, but it is correctable with a minor surgery (a lingual frenectomy). Ankyloglossia is a common congenital malformation, although there is significant amount of disagreement as to how prevalent it is. Estimates ranging widely, from 1% to 25% of births (potentially a lot of surgical bills). Similarly, there is disagreement as to how severe the congenital disorder must be before surgical intervention becomes necessary, and this disagreement has been going on for over 75 years.

Patient with large right Pharyngeal Cleft Cyst protruding from neck, prior to excision” by BigBill58 is licensed CC BY SA 4.0Branchial cleft cyst
Branchial cleft cysts form when incomplete fusion of neighboring pharyngeal arches leaves the remnant of a pharyngeal groove. These usually form a painless mass in the neck, until an infection occurs. They may be left untreated, or may be removed by surgery. This involves removing the extraneous ectodermal (epithelial) tissue trapped deeper in the neck. Whether surgery is or isn’t performed may depend on how close the cyst is to the carotid artery, internal jugular vein or facial nerve.
Rathke’s cleft cyst
Similar to branchial cleft cysts, a cyst may form from incomplete obliteration of Rathke's pouch during formation of the pituitary gland. This leads to a mucus-filled cyst near the anterior pituitary. Due to its location, it may put pressure on the optic chiasm, leading to visual disturbances, otherwise it is asymptomatic. Drainage is the preferred treatment over removal, owing to how close it is to the pituitary gland.
< Chapter 6 * navigation * Chapter 8 >
Chapter review questions
The process by which animals and plants grow and change.
Slowly change shape
The developmental history of a differentiated cell traced back to the embryonic cell from which it arises.
A series of bony or cartilaginous curved bars along the pharynx, supporting the gills of fish and amphibians.
A similarity due to shared lineage between a pair of structures or genes in different taxa.
A series of externally visible anterior tissue bands lying under the early brain that give rise to the structures of the head and neck.
The mucous membrane lining the inside of the mouth. It is a stratified squamous epithelium, named the oral epithelium, and an underlying areolar connective tissue named the lamina propria.
deoxyribonucleic acid is the hereditary material in humans and almost all other organisms.
The shape (or form) of.
To state again, or to repeat.
The development of an organism or anatomical or behavioral feature from the earliest stage to maturity.
The history of the evolution of a species or group, especially in reference to lines of descent and relationships among broad groups of organisms.
Five swellings that appear on the face in the fourth week
When two or more cells, tissues or organs join to become one.
Bilateral pairs of blocks of mesoderm that form along the rostral-caudal axis.
A temporary group of cells that arise from the embryonic ectoderm, and in turn give rise to a diverse cell lineage—including melanocytes, cranio-facial cartilage and bone, teeth and periodontal tissue, smooth muscle, peripheral and enteric neurons and glia.
Or the globular process, is a mass of tissue formed by the merging of the median nasal processes, fated to become the philtrum and pre-maxilla.
Reorganization or renovation of existing tissues, either physiological or pathological. The process can either change the characteristics of a tissue such as in blood vessel remodeling, or result in the dynamic equilibrium of a tissue such as in bone remodeling.
The anterior end of the primitive gut tube, derived from yolk sac endoderm, not yet connected to the oral cavity.
he most exterior of the three primary germ layers formed in the gastrula, it gives rise to the epithelial tissue covering the body and the CNS.
A depression between the brain and the pericardium in an embryo, and is the precursor of the mouth and the anterior lobe of the pituitary gland.
Inward folding of an epithelium caused by interstitial growth.
The region where the ectoderm and endoderm come into direct contact with each other constitutes a thin membrane, which forms a septum between the primitive mouth and pharynx.
A phase early in the embryonic development during which the single-layered blastula is reorganized into a multilayered structure known as the gastrula.
The innermost of the body's 3 embryonic tissues that form during gastrulation, it becomes the inner lining of hollow organs.
The middle of the three embryonic layers that develop during gastrulaton, mesoderm becomes most of the body's muscle and connective tissues.
A group of 235-300 related genes that code for a transcription factors, which control the activity of other genes involved in development, including directing the formation of limbs and organs along the anterior-posterior axis.
The embryonic process in which one group of cells directs the development of another group of cells.
The process of converting DNA into an mRNA copy
A sequence of nucleotides in DNA that encodes the synthesis of a gene product, either RNA or protein.
A substance whose non-uniform distribution governs the pattern of tissue development and pattern formation.
The three layers of cells that arise from gastrulation: endoderm, mesoderm and ectoderm.
Outward-folding of an epithelium caused by interstitial growth.
An ectodermal groove between two pharyngeal arches.
An endodermal pouch between two pharyngeal arches on the internal surface of the embryo.
The type or types of cell(s) a stem cell can possibly differentiate into in the future, determined by which genes are methylated and stored around histones, or free to be transcribed.
The branch of biology and medicine concerned with the study of embryos and their development.
The folding process in vertebrate embryos, which includes the transformation of ectoderm into the neural tube.
A process by which epithelial cells lose polarity and cell-cell adhesion, and gain migratory and invasive properties to become mesenchymal stem cells, this normally occurs during embryonic development and wound healing.
The embryonic precursor to the central nervous system.
When one cell begins to look different from another. This process involves limiting cell fate by altering gene transcription to become more specialized.
A subset of mesenchymal stem cells derived from neural crest cells rather than mesoderm, or mesoderm-derived mesenchymal stem cells induced by neural crest morphogens to adopt a more neural fate.
The process that results in an increase of the number of cells, and is defined by the balance between cell divisions and cell loss through cell death or differentiation.
The first branchial arch of the vertebrate embryo which in humans develops into the lower lip, mandible, masticatory muscles, and anterior tongue.
A process that grows off the mandibular arch on each side and forms the lateral part of the upper lip, cheek, and upper jaw except the pre-maxilla.
Tissue on the inner side of each nasal pit merge into the inter-maxillary segment and form the philtrum, crest and tip of the nose, and merge with the maxillary processes.
Tissue on the lateral sides of the nasal pits that form the alae of the nose.
An embryonic tissue composed of undifferentiated mesenchymal stem cells and mucous ground substance.
Copying DNA into a functional product, such as a RNA and/or protein. Controlled by the activity of transcription factors binding to gene promoter regions to recruit RNA polymerase.
a family of enzymes that catalyse the degradation of hyaluronic acid.
A very large glycosaminoglycan distributed widely throughout connective tissue ECM, epithelial, and neural tissues.
Gel-like substances in the ECM, composed of water held in place by large molecules (proteins, glycoproteins and glycosaminoglycans).
Cell Adhesion Molecules: proteins located on the cell surface involved in binding with other cells or with the extracellular matrix.
Trans-membrane structure specialized for cell-to-cell adhesion.
A trans-membrane protein which allows cells to bind to the ECM protein fibronectin, it is involved in cell adhesion, growth, migration, and differentiation.
A large glycoprotein found in the ECM which binds to integrin proteins on the cell surface, involved in cell adhesion, growth, migration, and differentiation.
Mesenchyme tissue that contains neural crest cell derivatives.
Apiece of cartilage from which the mandibles (lower jaws) of vertebrates evolved, originally the lower of two cartilages which supported the first branchial arch in early fish.
The formation of bone tissue from a cartilage model
Programmed cell death
vertical indentation in the middle area of the upper lip, extending from the nasal septum to the tubercle of the upper lip.
The second pharyngeal arch.
Sites within lymphoid organs where mature B cells proliferate and differentiate.
Small bumps that give rise to bigger structures such as hair follicles and teeth.
Fibroblast Growth Factors are a family of morphogens involved in a wide variety of processes, including important roles in development and tissue regeneration, especially connective tissues.
Undifferentiated or partially differentiated cells that can differentiate into various cell types, and proliferate to produce more of the same stem cell.
An epithelium composed of one layer of square cells
A polarity axis that organizes cells in the plane (side-to-side) of the tissue.
Signaling molecules first identified for their role in carcinogenesis, then for their function in embryonic development, including body axis patterning, cell fate specification, cell proliferation and cell migration.
A small sac-like cavity in a gland, surrounded by secretory cells.
Epithelial cells found in glands, containing smooth muscle actin allowing them to contract and expel secretions of exocrine glands.
Highly basic proteins found in nuclei that compact DNA into a denser form, unavailable for gene transcription.
Methyl groups added to DNA which change the activity of a gene without changing the sequence, typically repressing gene transcription.
An evagination at the roof of the developing mouth in front of the oropharyngeal membrane, which gives rise to the anterior pituitary (adenohypophysis).
Cells derived from ectoderm fated to develop into the CNS or neural crest.
The embryonic form of the oral cavity and nasal cavities before they are separated.
The roof of the mouth, separating the oral cavity and nasal cavities.
The pre-maxilla, includes that portion of the alveolar ridge containing the four incisors.
The portion of the hard palate formed by the growth of two shelves off the maxillary process medially and their mutual fusion in the midline.
The portion of the hard palate formed by the growth of two palatal shelves medially and their mutual fusion in the midline.
Bone Morphogenetic Proteins are a group of signaling molecules, initially discovered for their ability to induce bone formation, now known to play crucial roles in all organ systems.
The oral opening of the nasopalatine canal, located in the maxilla at the junction of the medial palatine and incisive sutures.
The stratified squamous epithelium of the oral mucosa.
A ridge running across the palate, from the palatine uvula to the incisive papilla.
The dense irregular connective tissue layer found below the oral mucosa (or mucosa of a hollow organ), homologous to the reticular layer of the dermis.
A swelling situated in the midline of the floor of the pharynx between the mandibular arch and of the second branchial arch that contributes to the formation of the anterior part of the tongue.
Two swellings on the floor of the primitive pharynx which, along with the tuberculum impar, form the anterior 2/3rd of the tongue.
A swelling that forms from the second pharyngeal arch, it quickly gets overgrown by the 3rd and 4th arch during development of the tongue.
A line that divides the dorsum of the tongue into symmetrical halves, from the anterior tip to the foramen caecum,
V-shaped groove separating the anterior two thirds of the tongue from the posterior third and containing the circumvallate papillae.
A small fold of mucous membrane extending from the floor of the mouth to the midline of the underside of the tongue.
The small depression at the border between the anterior (oral) and the posterior (pharyngeal) portions of the tongue. It is the point from which the thyroid gland formed by invagination.
Epithelia that have more than one layer of cells, with the cells at the apical surface having a flat shape.
The top surface of the tongue, containing lingual papillae and taste buds.
The bottom surface of the tongue, which contains no lingual papillae.
A terminally differentiated epithelial cell capable of synthesizing large amounts of the protein keratin within its cytoplasm.
The areolar connective tissue layer of the oral mucosa (or hollow organ), homolgous to the papillary layer of the dermis.
(of a disease) not harmful in effect, (of a tumor) not malignant.
Depressions of the lower lip.
The presence of pits and possibly associated fistulas in the lips.
A medical condition that is present at or before birth, which can be acquired during development or from the genetic make up of the parents.
an opening or split in the upper lip that occurs when there is incomplete fusion of the maxillary process(es) with the inter-maxillary segment.
On one side
On both sides
An opening or split in the roof of the mouth that occurs when the palatal shelves and/or primary palate fail to fuse completely.
A protein that controls the rate of transcription of DNA to mRNA, by binding to a specific DNA sequence.
A partially unfused cleft palate, where at least some of the bone portion is intact.
A fully unfused cleft palate, where no bony connection is made.
DNA molecule packaged into thread-like structures found during mitosis, visible under a light microscope.
A condition where the bone and cartilage that divide the nasal cavity of the nose in half is significantly off center, or crooked, making breathing difficult.
A loose connective tissue composed of fibroblasts and other cell type, plus ground substance and all three fiber types.
A surgical procedure to straighten the bone and cartilage of the nasal septum.
Cosmetic surgery that alters the appearance of the nose.
A harmless, painless bony growth located on the roof of the mouth.
Abony growth in the mandible along the surface nearest to the tongue.
In the phrase nature vs nurture, nurture means environmental factors that occur after fertilization, not DNA inherited from our parents.
In the phrase nature vs nurture, nature refers to genetics (heritable traits).
A congenital malformation that may decrease the mobility of the tongue caused by an unusually short lingual frenulum.
A fluid-fille sac causing swelling in the upper part of neck anterior to sternocleidomastoid.

