11 Periodontium development
- Overview
- Cementogenesis
- Development
- Types of cementum
- Periodontal ligament
- Cells
- Fiber groups
- Alveolar bone
- Gingiva
- Clinical considerations
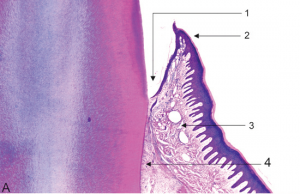
"Histological section of an incisor tooth of a Rhesus monkey displaying the elements of the periodontal apparatus" by András Mihály1 and Eszter Mihály is licensed under CC BY 4.0Overview
The periodontium includes cementum, PDL, alveolar bone and gingival tissues. The gingiva contain a stratified squamous epithelium← which develops from the ectoderm of the pharyngeal arches←. Underlying connective tissue layers develop from mesoderm (as does most of the skull). The ECM of these tissues contains collagen← and elastic fibers. Cementum, PDL and alveolar bone develop from the neuro-mesenchymal stem cells of the dental sac. These three tissues share extracellular components, thanks to their shared lineage, making for a strong connection between them. Like mesoderm-derived connective tissues, these tissues have a lot of collagen, but instead of elastic fibers, these tissues produce a special protein fiber type called Oxytalan fibers. Oxytalan fibers are found in a few other places in the human body, such as the aorta, whose lining is also derived from neural crest cells←. HERS← plays an important role in the induction of these tissues, even if it is mostly fated to undergo apoptosis←.
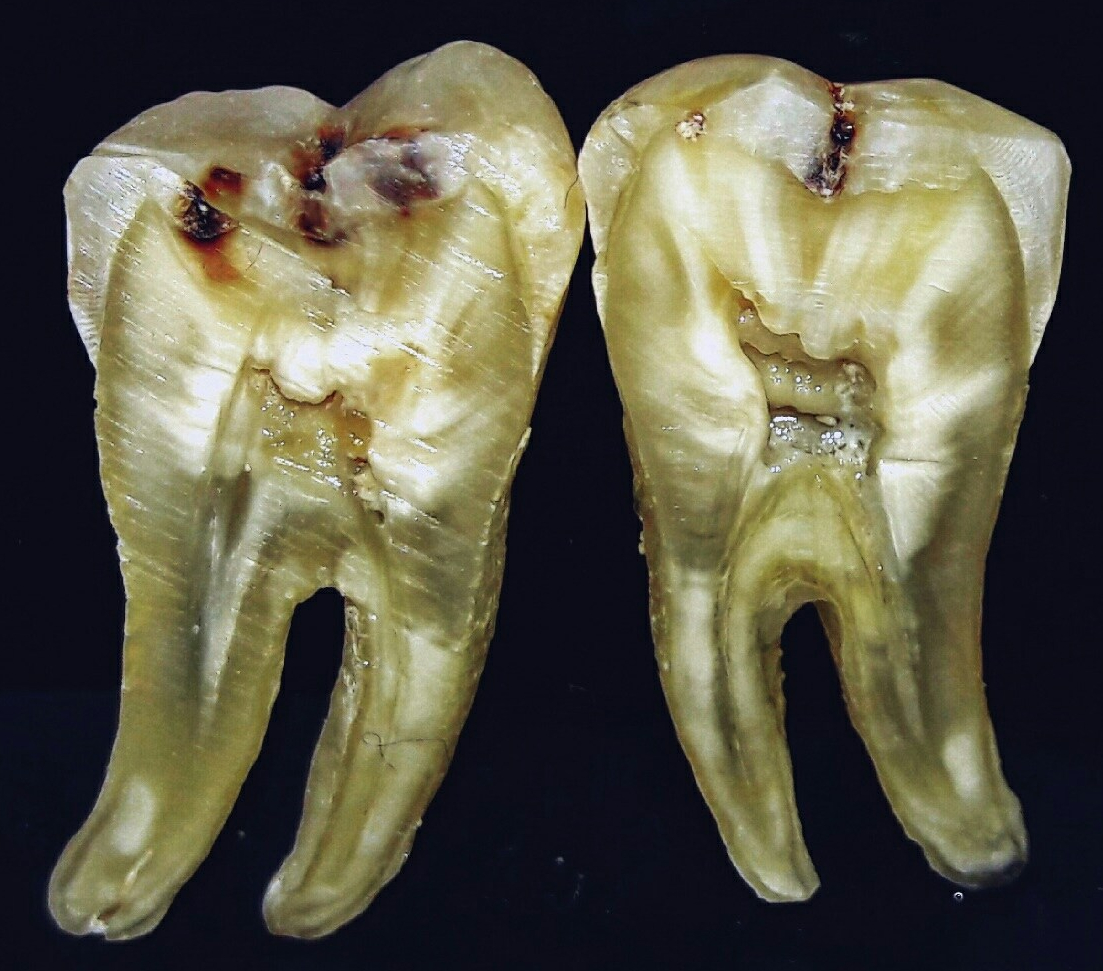
"What is inside a human tooth" by Mohamed A.M. Ahmed is licensed under CC BY-SA 4.0 / croppedCementogenesis
Cementum forms a thin layer on the roots of teeth, attaching the teeth to the alveolar bone by way of the PDL. The mineral content of cementum is lower than that of dentin, but not enough to make it appear different from dentin on a radiograph. The surface of cementum will feel grainier than enamel when explored with instruments.
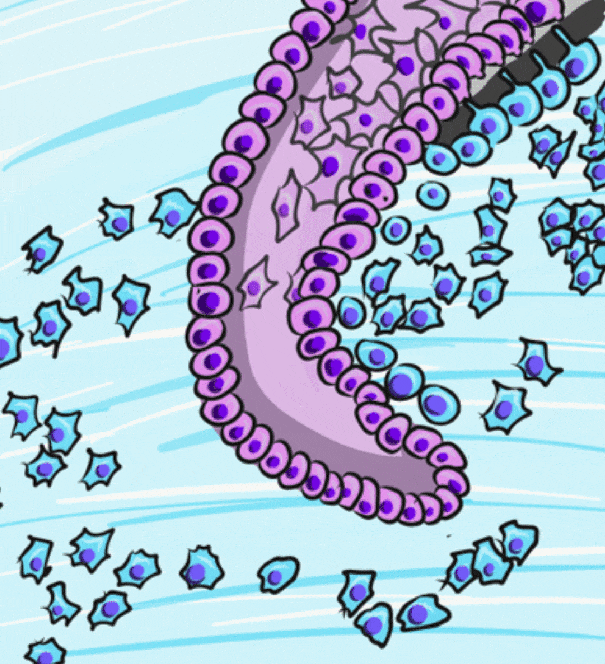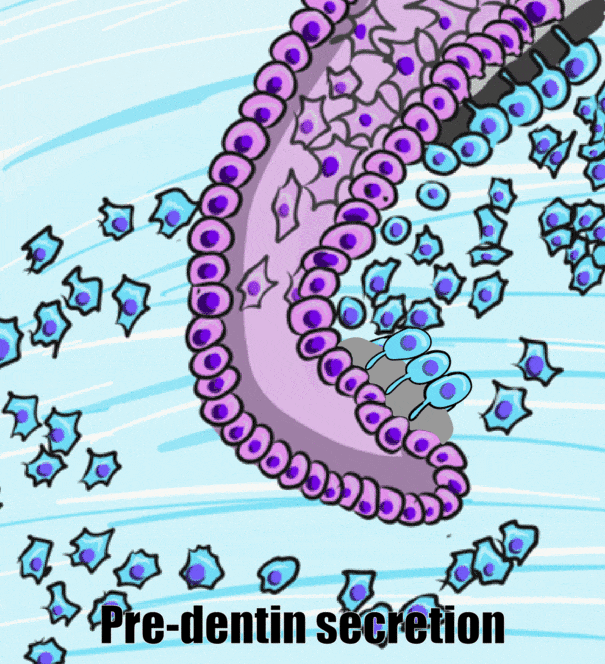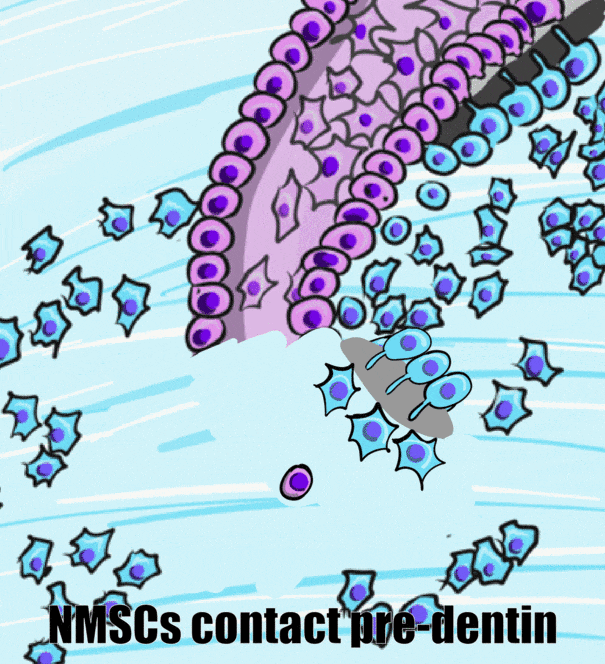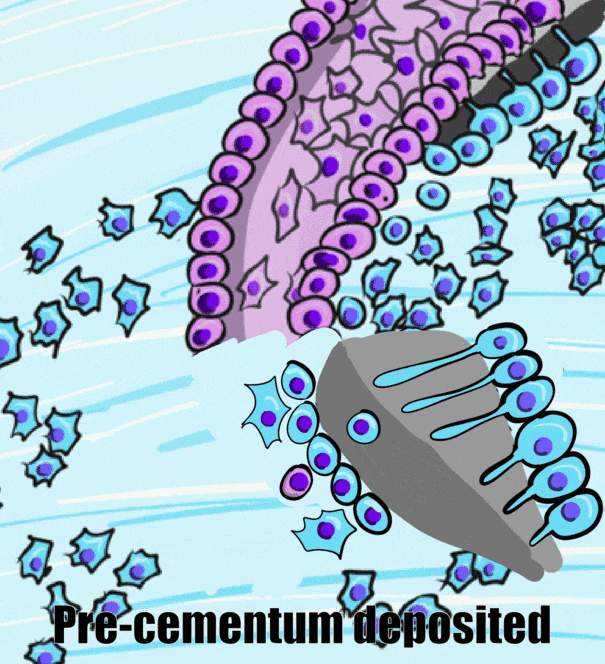
After crown formation is nearly complete, HERS← grows apically, separating the dental papilla from the dental sac. The IEE of HERS induces the differentiation← of odontoblasts, which begin dentinogenesis←. Without stellate reticulum, the IEE cannot be reciprocally induced to form ameloblasts. Instead, the IEE and OEE secrete morphogens← onto the surface of dentin, including members of the FGF and Wnt families, but after that, these cells mostly undergo apoptosis←. A few of the epithelial cells of HERS may undergo an epithelial-to-mesenchymal transition← and differentiate into cementoblasts. However, most cementoblasts arise differently. With HERS out of the way, pre-dentin← made by root odontoblasts contacts neuro-mesenchymal stem cells of the dental sac. Contact with pre-dentin and BMP morphogens secreted from the dental papilla induces the neuro-mesenchymal stem cells of the dental sac to become cementoblasts. These cells, like odontoblasts, align next to one another, behaving more like a tissue derived from ectoderm (an epithelium) than a connective tissue.
Cementogensis is the process of forming cementum. Cementoblasts secrete the protein components of cementum. This immature matrix is commonly referred to as cementoid, which breaks from the pattern we used for pre-enamel and pre-dentin, or it may be referred to as pre-cementum. Layers of pre-cementum are laid down appositionally, and soon mineralize, at which time it is called cementum. Some cementoblasts become trapped in cementum, after which they are referred to as cementocytes. Other cementoblasts remain near the surface of the cementum. These cells continue to lay down layers of pre-cementum throughout life, and can become more active during times of injury and repair to cementum. Because of the shared lineage between cementoblasts and odontoblasts, and the similarity of their ECM, the CDJ is less distinct than the DEJ. In fact, the CDJ was once considered to be imaginary (reviewed here).
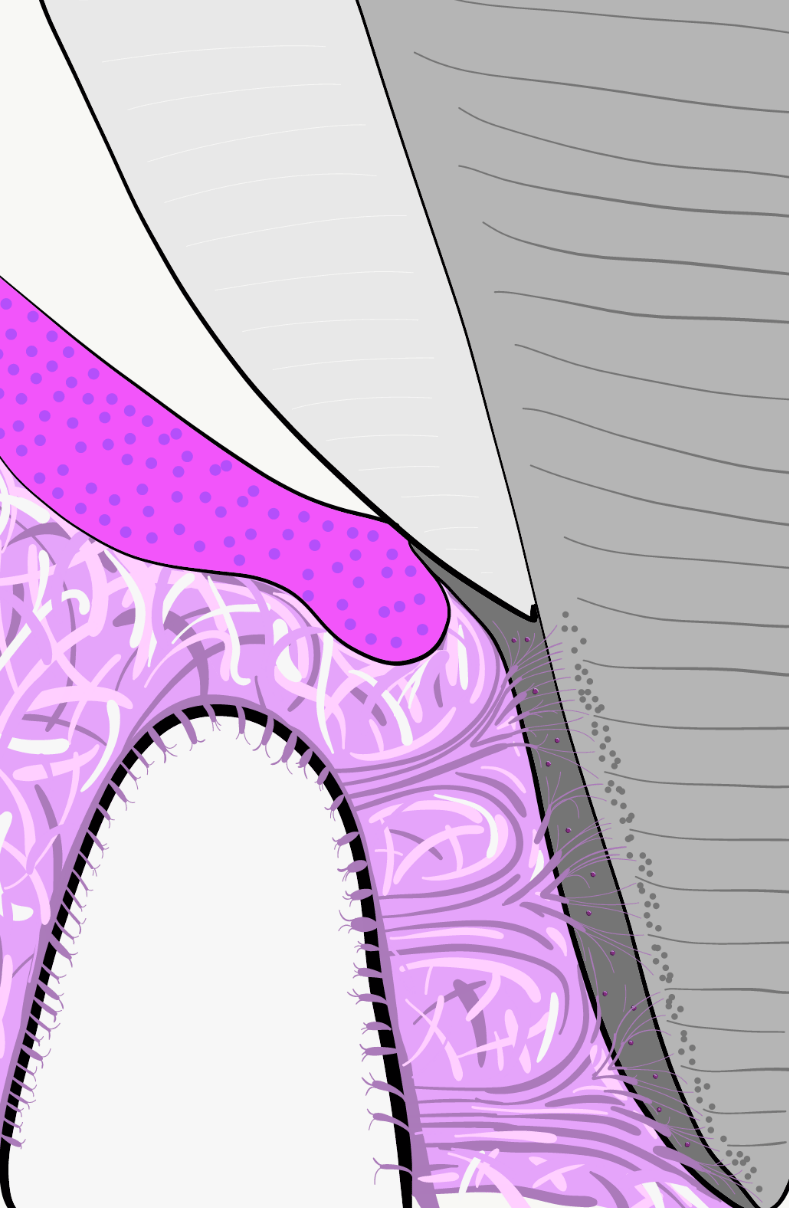
The major protein in cementum is collagen←. Collagen fibers extend out of cementum into Tomes’ granular layer of root dentin, and in the other direction become the collagen fibers of the PDL. Collagen fibers of the PDL, in turn, become Sharpey’s fibers← embedded in alveolar bone. Rather than thinking about alveolar bone, PDL, cementum and dentin as distinct tissues bonded to each other, think of their borders as a gradient, thanks to their shared lineage. This makes for a stronger connection than bonding 4 separate tissues, especially ones that are too thin for large rete pegs← and dermal papillae-like digitations. Other proteins secreted by cementoblasts include two glycoproteins (bone sialoprotein and osteopontin) which help collagen adhere to calcium hydroxyapatite crystals. Cementoblasts also secrete enzymes that catalyze the formation of crystals, similar to those active in dentinogenesis←.
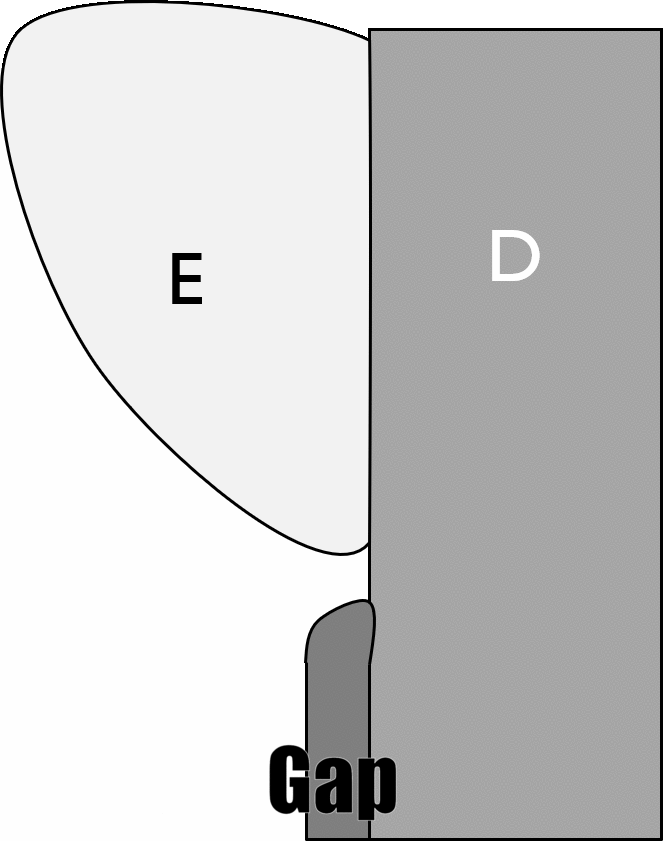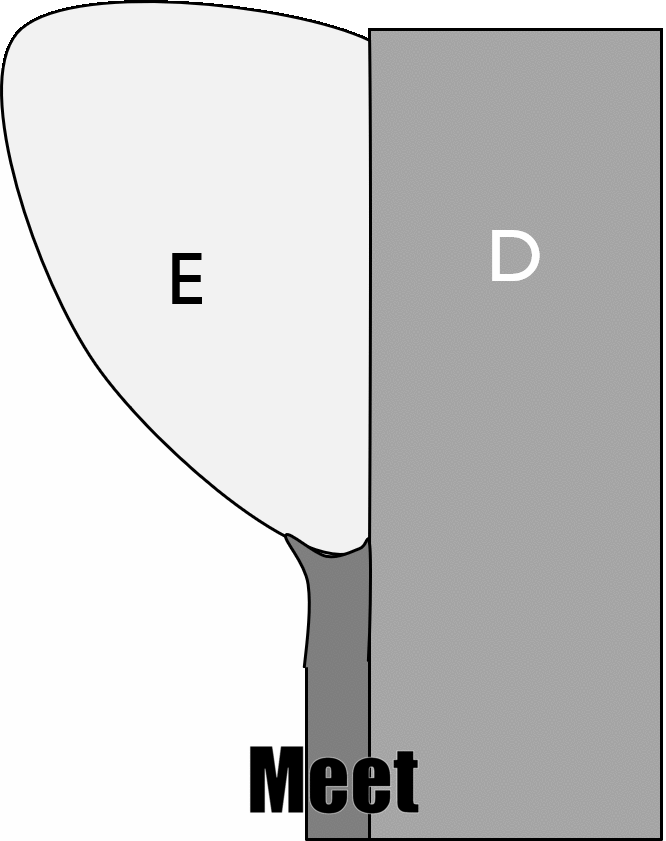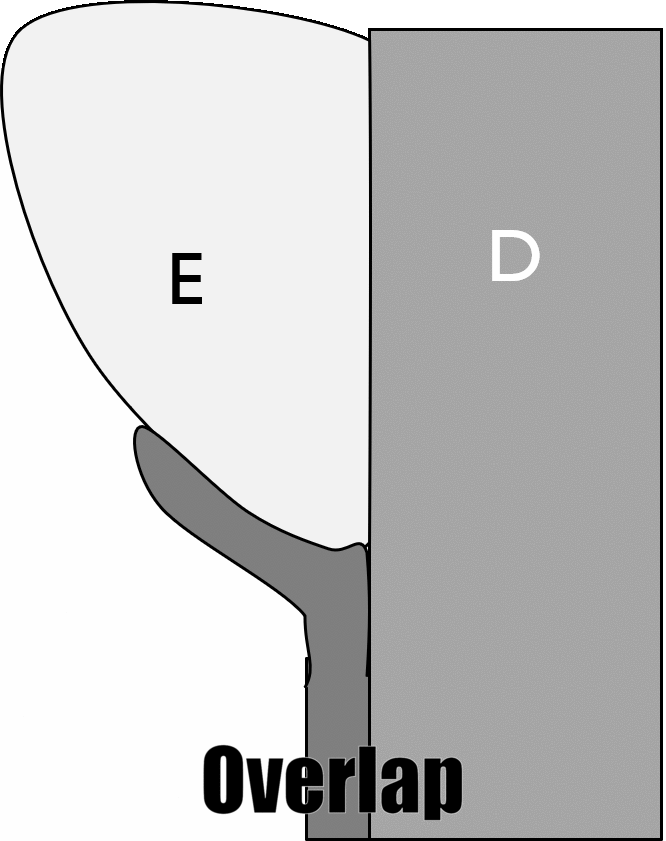
Types of cementum by area of the root
For most teeth, the apical one third of the root contains cellular cementum over acellular cementum. More cervical regions contain only acellular cementum. Most of the time, roughly 60%, cementum extends over the enamel a short distance. About 30% of the time, cementum completely covers root dentin, all the way up to the enamel. Roughly 10% of the time there is a gap between the cementum and enamel, with underlying dentin exposed. Rarely, about 1% of the time, enamel may overlap cementum (rare because amelogenesis begins much earlier than cementogenesis). These percentages are not for individual teeth, but for any area of the cervical region on a single tooth. Therefore, a single tooth may have all patterns. The gap pattern is the most significant because of the increased risk of dentin hypersensitivity←. Cementum found overlapping enamel contains no collagen← fibers, and does not connect to the PDL.
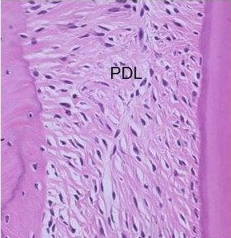
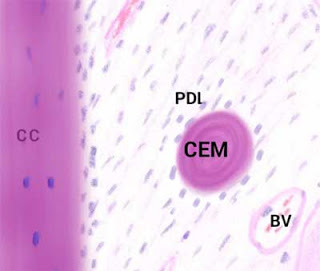
"Immunohistochemistry of CXCL12 in PDL of rat molars” by Yashiro Y et al is licensed under CC BY-SA 4.0 / croppedPeriodontal ligament development
The PDL, like other ligaments, is a dense regular connective tissue←, composed primarily of collagen← fibers in the ECM, plus the special oxytalan fibers. These fibers are made by fibroblasts that differentiate from neuro-mesenchymal stem cells of the dental sac. In figure 11.6, fibroblast nuclei stain purple. From this image, you should be able to determine which side of the PDL is anchored to bone tissue versus anchored to cementum. Look at either border, are there cells within lacunae or not? The pink color is mostly collagen in all 3 of these tissues.
Sample embedded in tooth preservation cocktail and nourishment media” by PM Sunil et al is licensed under CC BY-NC-SA 4.0Like other ligaments, the PDL contains osteoblasts and osteoclasts (mostly at the border with alveolar bone), but unlike other ligaments, the PDL also contains cementoblasts and a unique population of stem cells←. The stem cells are different from mesenchymal stem cells, and may be referred to as Periodontal Ligament Stem Cells (PDLSCs). These stem cells can differentiate into fibroblasts, osteoblasts, odontoblasts, cementoblasts, cementoclasts or odontoclasts given the correct morphogen←. This allows the PDL to play a role in the remodeling and repair of bone, cementum and dentin. Whether these stem cells are different from the neuro-mesenchymal stem cells found in the dental sac and dental papilla is unclear. Their unique properties, however, make them useful: stem cells from extracted teeth can be used to promote the healing of many other (non-tooth) tissues.
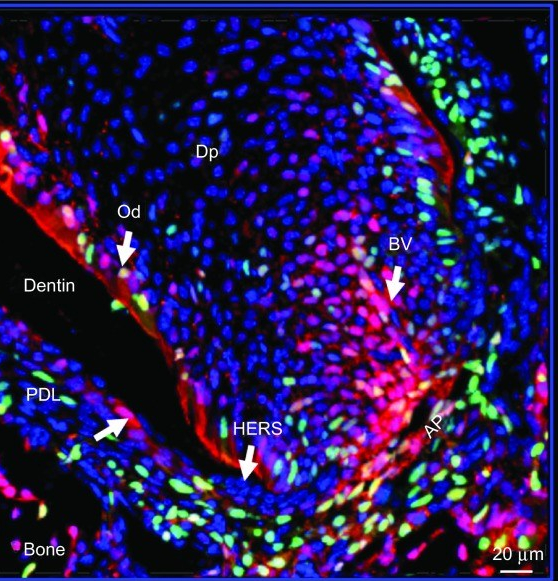
Lineage studies by mapping Cre activity in the root region of a second molar in a 2-week-old mouse by confocal microscopy using the Olympus FV 1000.” by Rakian A, et al is licensed under CC BY-NC-ND 4.0Unlike cementum and dentin, the PDL is vascular. The PDL is significantly more vascular than other ligaments, thanks to its lineage. PDL fibroblasts secrete morphogens← inlcuding vascular endothelial growth factor (VEGF) to promote angiogenesis. The expression of the VEGF gene is controlled by morphogens of the BMP and FGF families. BMPs induce the differentiation of odontoblasts and osteoblasts, whgingival ile FGF induces fibroblast differentiation. Both morphogen signals are necessary to specify PDL fibroblast differentiation. What also makes PDL fibroblasts different from other fibroblasts is that they have a neuro-mesenchymal lineage. They have a higher capacity to undergo renewal (new ones forming from PDL stem cells←) and trigger tissue repair than fibroblasts in other ligaments. In other ligaments, fibroblasts develop from mesoderm, and are induced to differentiate from mesenchymal stem cells by FGF alone.
Oddly, the tissues that can best be repaired by cells from the PDL are bone and cementum, not the PDL itself. Keep this in mind as we discuss differentiation of the PDL. The first important concept is PDL fibroblasts are not like other fibroblasts. In addition to VEGF, PDL fibroblasts express genes shared by other cells with a neuro-mesenchymal lineage. For instance, PDL fibroblasts share features with cementoblasts, expressing RUNX2 and pre-cementum proteins. PDL fibroblasts also have similarities to neural tissue, expressing Neuronal Cell Adhesion Molecules (NCAMs)← and N-cadherin (a neuronal desmosome← protein) (scientific review article). These genetic details are less important to us, what is important is the PDL develops from neuro-mesenchyme.
One more unique feature of PDL fibroblasts is their ability to participate in an immune response. Unlike other ligaments, the PDL is often exposed to bacteria and bacterial toxins. In response to certain inflammatory signals, PDL fibroblasts down-regulate expression of genes involved in bone remodeling and behave more like white blood cells (scientific article for further reading). This is not entirely unexpected given their lineage. Neural crest cells guide the development of the thymus from the 3rd pharyngeal pouch (The thymus is where T cell lymphocytes develop).
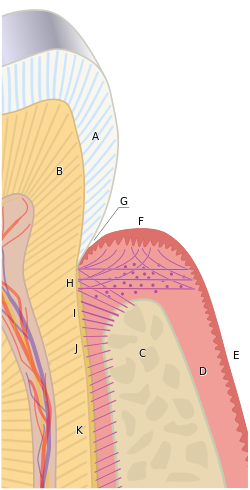
"the periodontium” by Goran tek-en is licensed under CC BY-SA 4.0Development of the PDL begins during tooth eruption←. PDL fibroblasts secrete collagen← fibers that become embedded in cementum and extend outwards (principal fibers). This process begins at the CEJ and continues apically as the root grows. The other ends of collagen fibers are embedded within alveolar bone later, activated by tooth occlusion. By waiting to anchor one end of the fibers until tooth occlusion results in greater mobility of tooth roots during tooth eruption←. PDL collagen fiber bundles run in different directions, which can be classified as alveolar crest, horizontal, oblique, (peri) apical, inter-radicular (on multi-rooted teeth) and trans-septal. Gingival fibers are also collagen fiber bundles, but anchor cementum to the gingiva. These are also categorized based on their location and fiber orientation. Depositing collagen in the correct orientation requires morphogens← of the planar cell polarity← class. One example is a cell-surface membrane protein called CD44 which binds to hyaluronic acid←, collagen and fibronectin←. Similar morphogens are involved in the polarization of odontoblasts following their induction by the IEE. Changes to polarity morphogens are likely necessary for re-orientation of fibroblasts during tooth occlusion and the production of collagen bundles in different directions.
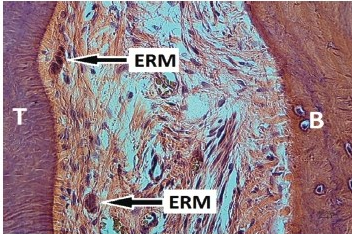
Photomicrograph of the periodontal ligament showing clusters of epithelial cell rests” by HD Miniggio and EJ Raubenheimer is licensed under CC BY-NC 4.0Remnants of HERS← may linger after root formation is finished. These cells, the Epithelial Rests of Mallasez, may be involved in repair of cementum and the PDL (the evidence is not definitive at this time). While these cells are epithelial, derived from ectoderm, they may undergo an epithelial-to-mesenchymal transition← and then differentiate← into cementoblasts or fibroblasts, if they receive the correct morphogens←. Evidence for this comes from studying transgenic mice.
Alveolar bone
The vault of the skull develops by intra-membranous ossification (including the maxilla). Thus, skull sutures contain dense connective tissue (unless they fully ossify). The base of the skull, on the other hand, develops by endochondral ossification←. The development of the mandible is even more complicated. First, neuro-mesenchyme forms two bands of cartilage (Meckel’s cartilage). The posterior portion is fated to become the ramus of the mandible (plus the malleus and incus bones), while the anterior portion disappears. Before the anterior portion disappears, the body of the mandible forms around it by intra-membranous ossification. Later, the condylar process, coronoid process and mandibular symphysis develop by endochondral ossification. That is how the skull forms. What is forms from does not match as nicely. Most bones develop from mesoderm, including the entire appendicular skeleton, plus most of the axial skeleton. The exceptions are the facial bones (including the hyoid) and inferior parts of the cranium, which develop from neuro-mesenchyme (the “new head”). In developmental biology classes, this is not a minor issue: ancient fish (either long extinct, or modern-day lampreys) have no lower jaw, they have seven branchial arches (pharyngeal arches←). Jawed vertebrates (gnathostomes), including humans and bony fish, remodel some arches into other structures, including a powerful mandible and ear parts. If you are curious, the lower jaw of a shark (and even the sturgeon) is not a mandible, it is Meckel’s cartilage (so sharks are a closer family member of yours than a lamprey, but more distant relative than a goldfish).
This brings us to alveolar bone. The alveolar ridge of both the mandible and maxilla develop from neuro-mesenchyme of the dental sac. Neuro-mesenchymal stem cells in this area are induced to differentiate into osteoblasts by morphogens← (including members of the BMP family). This in turn activates transcription factors such as members of the RUNX family and MSX homeobox gene← family. These transcirption factors up-regulate expression of bone-specific proteins. From a development standpoint, it is important to understand that the basal bone of the mandible and maxilla are induced by signals from Meckel’s cartilage, while alveolar bone develops from the tooth germ.
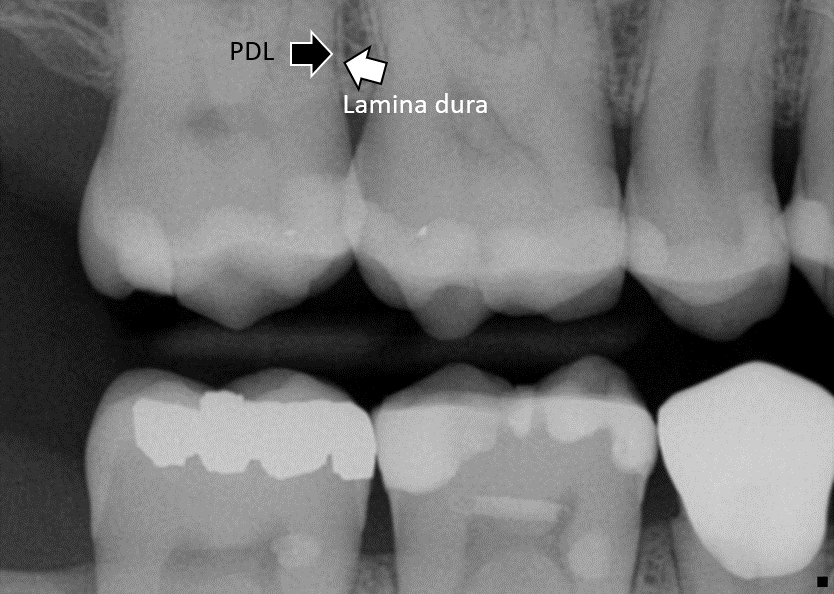
Alveolar bone contains numerous small Volkman’s canals, through which PDL fiber bundles insert into bone tissue. This is the same name used for the perforating canals that run perpendicular to Haversian canals in compact bone, only those Volkman’s canals contain blood vessels. Like all bones, the outer portion of alveolar bone is compact bone and below that is spongy bone. The compact bone portion is referred to as the lamina dura on a radiograph, as it shows up more radiopaque than the spongy bone deep to it (or the PDL superficial to it). The inter-dental septum is a triangular-shaped area between two teeth, which should be the height of the alveolar crest. The inter-radicular septum is the portion of alveolar bone between roots
Gingival development
The oral mucosa, including most of the gingiva, develop from ectoderm and mesoderm during embryonic development. Ectoderm forms the oral epithelium, while mesoderm forms the lamina propria and sub-mucosa. The exception is junctional epithelium←, which develops from a group of cells more specialized than ectoderm. During odontogenesis, ectodermal cells of the tooth germ separate from oral ectoderm. During tooth eruption←, it is the REE that develops into junctional epithelium.
Clinical applications
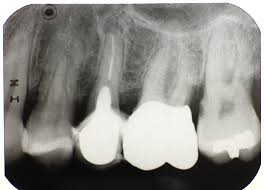
Periapical radiograph showing radiopaque halo around the root of tooth #36” (international numbering system) by Antonione Santos Bezerra Pinto is licensed under CC BY-NC-SA 4.0Hyper-cementosis
Because cementoblasts are located at the surface of adult cementum (within the PDL), cementum has the ability to undergo remodeling and repair. This makes cementum similar to dentin but different from enamel. Excessive cementoblast function can result in hyper-cementosis. This occurs more frequently at the root apex due to excessive occlusal forces on a tooth. It may also be caused by growth factor← disturbances, such as gigantism/acromegaly or Paget’s disease.
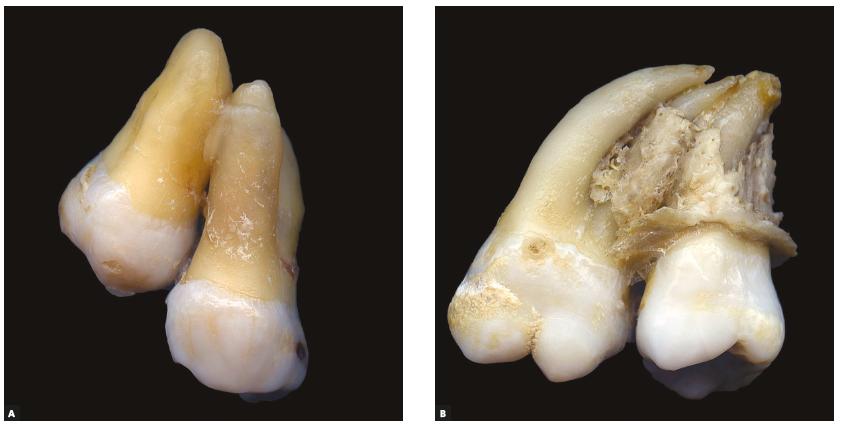
Second and third molars joined by cement, in teeth with and without hypercementosis” by Consolaro A et al is licensed under CC BY 4.0Concrescence
Enough hyper-cementosis can adhere two teeth together by the roots, which is called concrescence. This should illustrate one reason why a radiograph is necessary prior to tooth extraction. This most commonly occurs between the second and third upper molar.

Photograph showing root caries in 33 & 34” (international numbering system) by B Gupta is licensed under CC BY-SA 4.0Root caries
Gingival recession is worrisome in part because it exposes the roots of teeth to the environment that only enamel is exposed to under healthy conditions. Because of its lower mineral content, root caries spread quickly. Caries pass quickly to dentin, and if untreated, pulp. Dental pain may not arise until infection of the pulp sets in. If that happens, treatment is generally limited to tooth extraction, rather than prevention and maintenance. Root caries can be detected early with radiographs or use of a dental mirror and explorer.
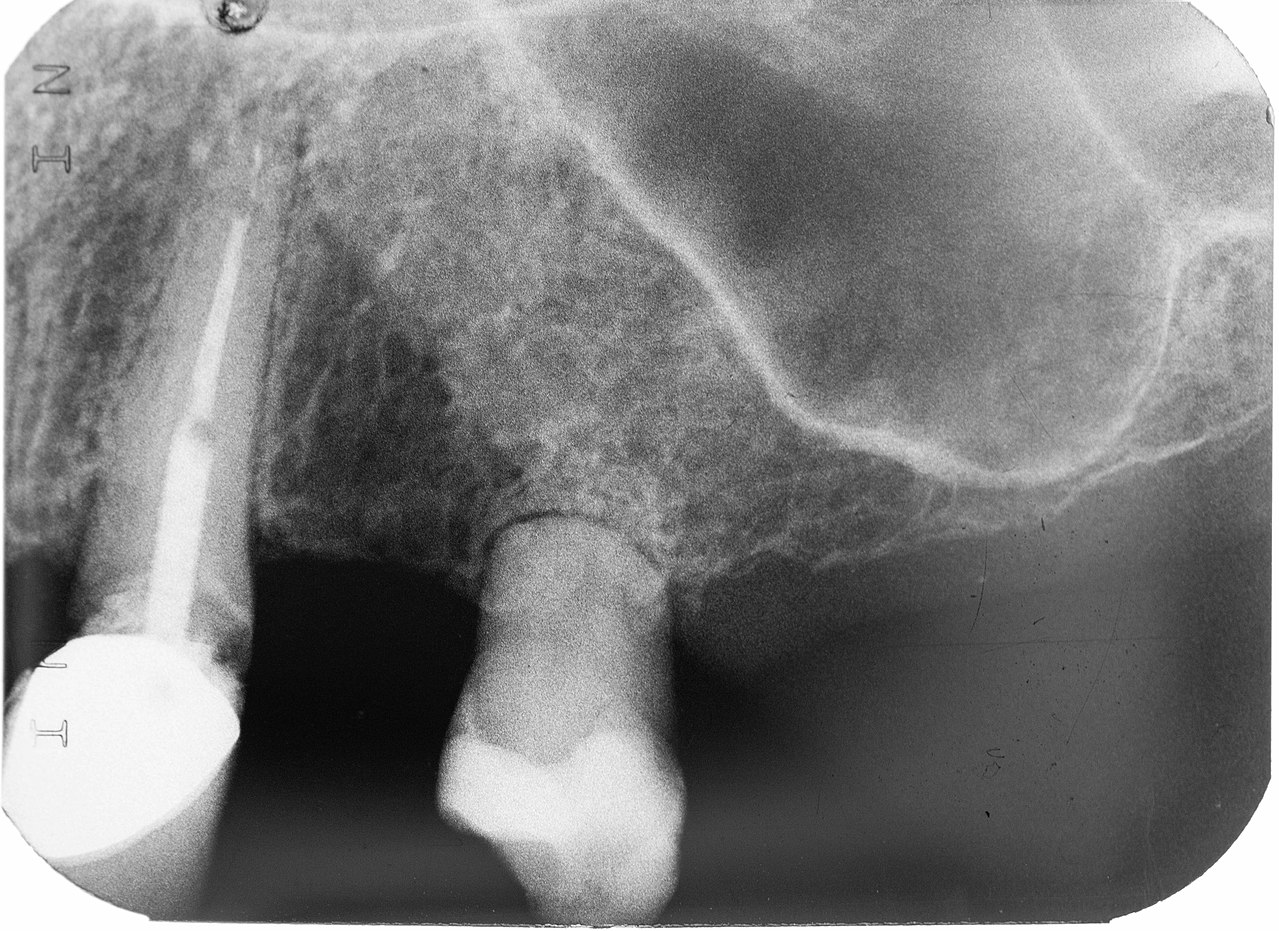
Own work” By Bin im Garten is in the Public Domain CC0Root resorption
Both dentin and cementum have the capability to be repaired in response to mild or moderate trauma. This is because living odontoblasts are located in the outer layer of the pulp, and cementoblasts in the PDL. If necessary, mesenchymal stem cells in the pulp or PDL may be induced to differentiate← and lay down more ECM. However, reparative cementum does not contain Sharpey’s fibers and does not contribute to tooth attachment to bone tissue.
Severe trauma, on the other hand, can induce the differentiation← of mesenchymal stem cells into cementoclasts or odontoclasts, leading to the loss of cementum and dentin. It is possible that the precursor to cementoclasts and/or odontoclasts may not be your average mesenchymal stem cell, but a related stem cell← with a different cell fate. Possible candidates include neuro-mesenchymal stem cells and PDL stem cells.
Mummery” by DRosenbachis licensed under CC BY 3.0Loss of dentin and cementum from the roots of teeth is root resorption. Loss of dentin from deep layers is internal root resorption. As dentin is lost, the space is filled in by vascular pulp tissue, possibly causing the tooth to have a more pinkish coloration. This tooth may be referred to as a pink tooth of Mummery (named for the anatomist who first described the condition). If internal root resorption is caused by chronic inflammation, removal of inflamed pulp by endodontic therapy← may halt the condition.
External root resorption is the loss of cementum and dentin from the superficial side of the root. External root resorption may be transient and resolve on its own. This occurs because cementum has a high regenerative capacity because of cementoblasts in the PDL. Chronic inflammation or tooth ankylosis may trigger more severe resorption, and replace lost tooth tissues with bone tissue.
For those into crime shows, corpses that have suffered a traumatic death (e.g. asphyxiation) or have spent time in a humid environment before being discovered may have pink teeth. This is due to ruptured pulp blood vessels causing bleeding into dentinal tubules (pdf download— warning: contains photos of a dead body).
Cementicles” by Mandana Donoghue is licensed under CC BY-NC-ND 4.0Cementicles
Masses of acellular cementum may develop in abnormal locations, especially later in life. These are referred to as cementicles. Cementicles may be free (within the PDL but unattached to the tooth), attached (on the surface of the layer of cementum) or embedded (once attached, but now surrounded by the cemental layer of the root). It is thought cementicles develop when cementoblasts encounter debris such as a blood clot (thrombus) lodged in a nearby capillary. This disrupts appositional growth of cementum and instead creates a nucleus around which a cementicle forms.

Tortoiseshell cat carrying her kitten up a flight of stairs. She grips the kitten's scruff in her teeth” by Margo Akermark is licensed under CC BY 2.0Tooth discomfort following endodontic therapy
After endodontic therapy←, the pulp—including nerve endings– is replaced with a non-living bio-compatible polymer. Nevertheless, tooth discomfort may occur. This is because the PDL is present and living. Besides functioning to attach the tooth roots to bone and gingival tissue, the PDL is involved in proprioception (kinesthesia), or the sensation of where the body is located. Nerve endings in the PDL relay information to the somatosensory cortex about where the teeth are located and when they experience occlusal forces. This is very important in reducing or avoiding occlusal forces during mastication and speech. The jaws exert the greatest amount of force the human body can generate, but also exhibit great control. It is routine for people to bite down through substances of varying density and thickness without teeth hitting one another thanks to a large region of somatosensory cortex interpreting information from the teeth.
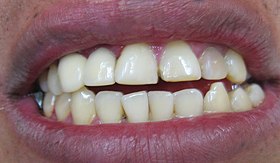
Class II human Molar relationship” by Dr. Vipin C. P. is in the Public Domain, CC0Mesial drift
Mesial drift (or physiological drift) is the tendency of teeth to migrate in a mesial direction with age. It is a natural phenomenon caused by the assymetrical remodeling of alveolar bone tissue. There are two major concepts relevant to mesial drift. One, alveolar bone undergoes more bone remodeling than other bone tissue. Two, this remodeling responds to occlusal forces: removing force causes bone loss to accelerate, adding force causes bone deposition to accelerate. When occlusal forces are assymetrical on a tooth, it leads to one side of the alveolar socket undergoing bone depostion and the other side bone resorption. Assymetrical remodeling, in turn, causes tooth movement (see Fig. 11.29 for a similar phenomenon). With age, teeth tend to wear down at their contact points, which leads to gaps between teeth, and a reduction in force on the gap side. Mesial drift causes teeth to move closer together, closing gaps as they form from wear. Too much movement can lead to crowding. Missing teeth can accelerate drift, causing neighboring teeth to lean lingually, changing the occlusal plane. This, in turn, can lead to abnormal forces on the temporo-mandibular joint, causing pain, discomfort and reduced range of motion.
Own work” by By Bin im Garten is licensed under CC BY-SA 3.0Loss of alveolar bone
The loss of alveolar bone leads to an uneven level of the interradicular septa or inter-dental septa. The consequence of this is reduced connection between tooth and bone, which can lead to tooth mobility and loss. This is often first observed in reduced opacity of the alveolar crest region(s) on a radiograph (Fig. 11.21). Under healthy conditions, alveolar bone—especially spongy bone—undergoes contant replacement by the remodeling unit←. Chronic inflammation, such as from periodontitis, inhibits the rate of osteoblast function without reducing the rate of osteoclast function. This is likely due to the fact osteoclasts are related to white blood cells, their lineage is from the bone marrow. White blood cells are more active during inflammation, unlike most other cells in the body.

Fenestration and dehiscence
With significant alveolar bone loss, portions of tooth roots may become exposed. A small window of root may become exposed, known as a fenestration (the Latin word for window). If the exposed root area connects all the way to the CEJ, the region is known as a dehiscence (a general term for a wound that cannot close). This is distinct from gingival recession or periodontal pockets←, where junctional epithelium← migrates to a region more apical on the root of the tooth, exposing cementum. A fenestration or dehiscence may still be covered by oral mucosa, but a lack of epithelial attachment means oral bacteria may come into contact with cementum and bone tissue, neither of which is designed to resist infection the way partially keratinized avascular epithelia are. This occurs much more frequently on the facial side than the lingual side.
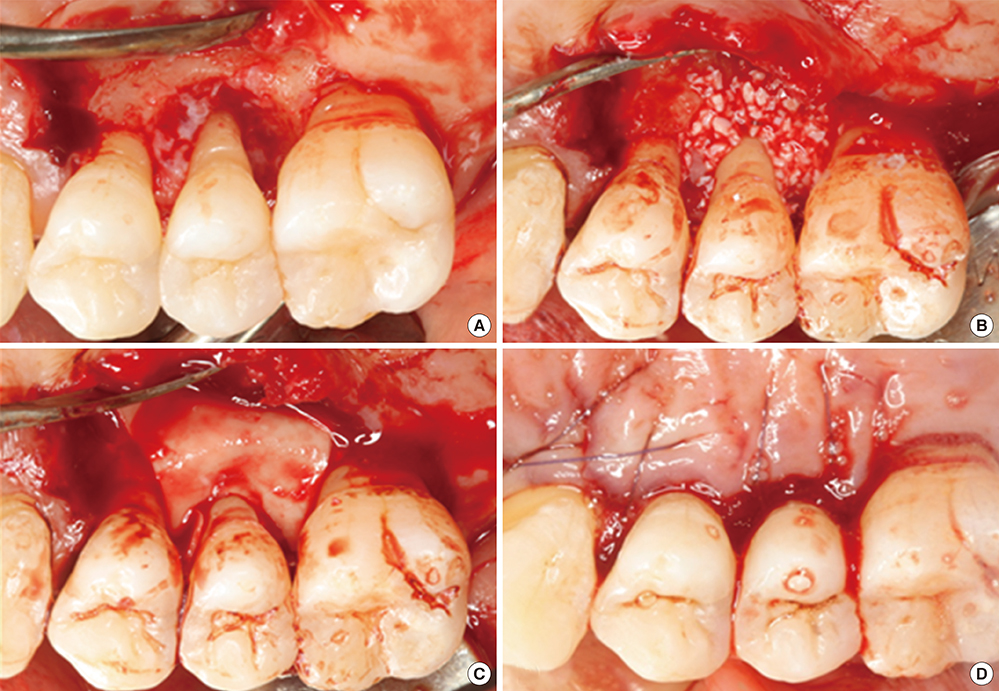
Chronic periodontitis, Collagen, Guided tissue regeneration” by Young Mi Chung is licensed under CC BY-NC-SA 4.0Guided Tissue Regeneration
There are many techniques that aim to stimulate the body to repair bone loss, including alveolar bone, by filling in the damaged area with a scaffold←. The proper scaffolding material allows mesenchymal stem cells to migrate through the damaged area and differentiate into osteoblasts. Surgeries involving this method are referred to as Guided Bone Regeneration or Guided Tissue Regeneration (GTR). The major difference between these procedures and a bone graft← is the use of a membrane to prevent neighboring tissue from growing into the damaged area.
First, a membrane must be placed over the injured area. Old-fashioned membranes, such as cotton gauze bandages, form a barrier between pathogens and exposed vascular tissue. Membranes can also keep other tissues, such as epithelia or scar tissue, from filling up the lost region with the wrong type of tissue. Cotton, however, does not contain molecules that bind to human integrins or CAMs, and therefore does not act as a scaffold. Without a scaffold, healing of the injury occurs from the edges of the wound. Newer materials may incorporate molecules covered in this book, and be recognized as a scaffold over which stem cells migrate. These molecules may include components of ECM, or morphogens←. Some morphogens promote the differentiation← of mesenchymal stem cells into osteoblasts, rather than fibroblasts (which produce scar tissue), others can be used to promote angiogenesis, speeding up the healing process.
One consideration when choosing a membrane is whether it is resorbable, or whether it will need to be removed (requiring another surgery). Both organic and synthetic polymers may be capable of being resorbed, or not. For instance, cotton is organic, but is not resorbed readily by the human body. Poly-lactic acid (PLA) membranes (which are also organic, but synthesized in a laboratory) are readily absorbed during the healing process and get replaced by human tissues. Resorption of large molecules may occur without the activity of human cells. For example, PLA slowly degrades when exposed to heat and UV light. Other polymers might be removed by human enzymes, such as matrix metalloproteinases. Guided tissue regeneration is conceptually very different from replacing damaged tissues with a bio-compatible but inert material, such as latex in endodontic therapy←, or a filling made of metal, porcelain or plastic. The goal is not to fill in a gap, but to promote the healing of a gap.
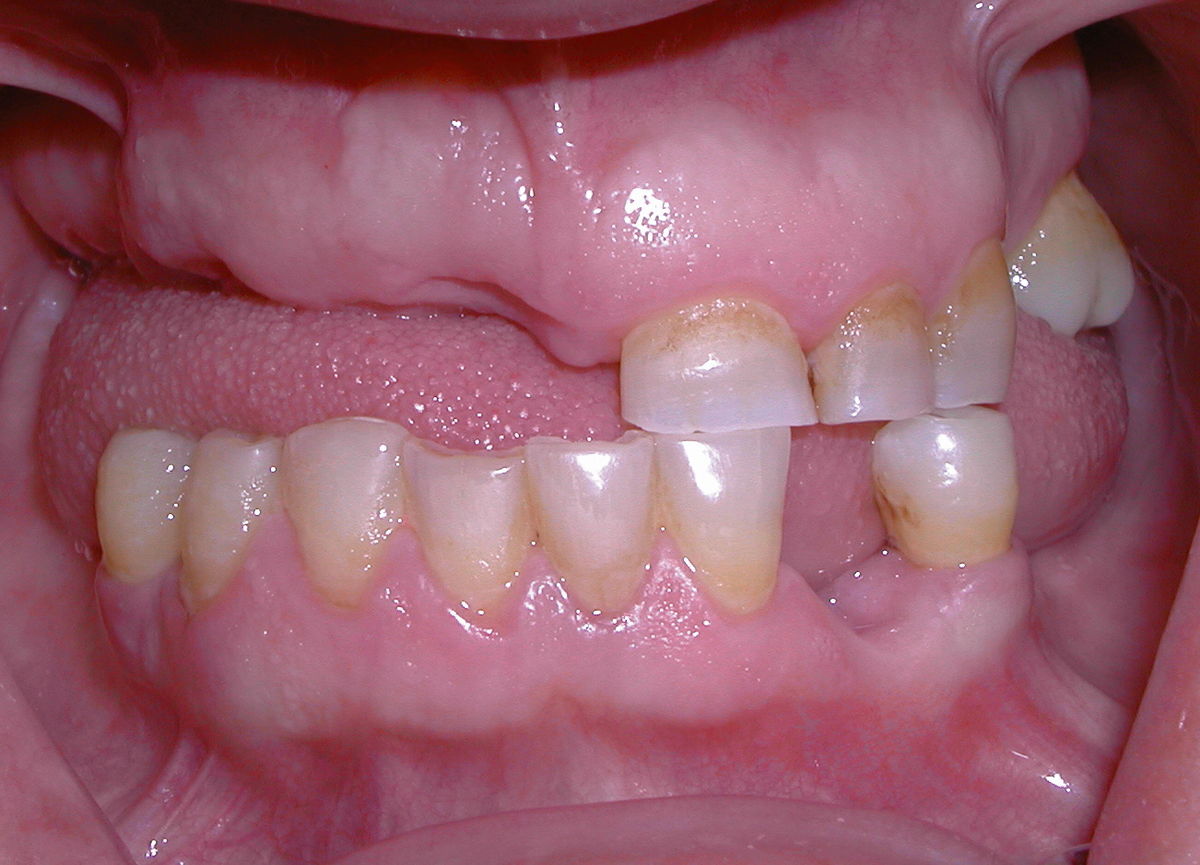
Own work” by JASFUS assumed in the Public Domain CC0Resorption of the alveolar ridge
Following tooth extraction or loss, regions of the alveolar ridge no longer anchored to PDL and tooth roots undergo resorption (basal bone is unaffected). This occurs because all bone tissue undergoes remodeling←, and a lack of tension slows down the activity of osteoblasts, but not osteoclasts. The opposite is why exercise maintains healthy bones. Chewing exercises the alveolar ridge.
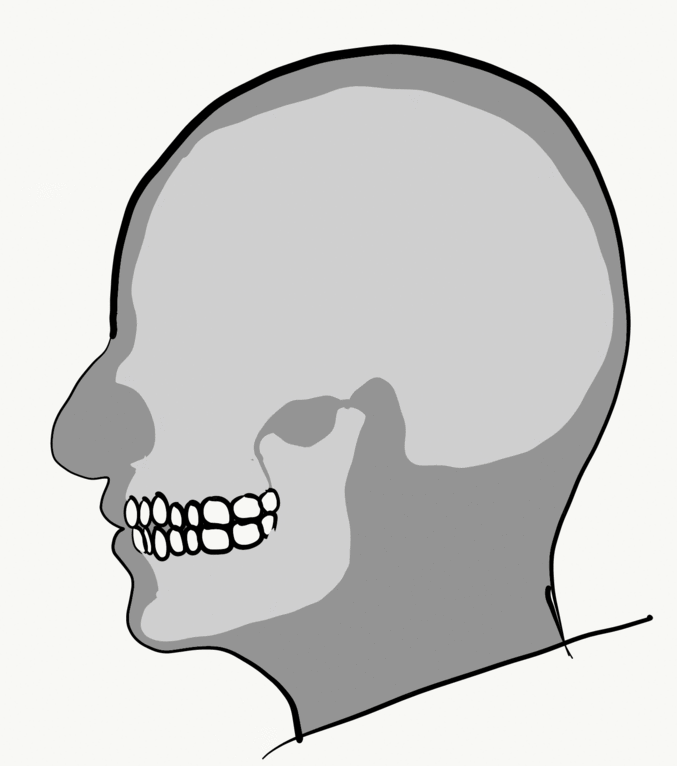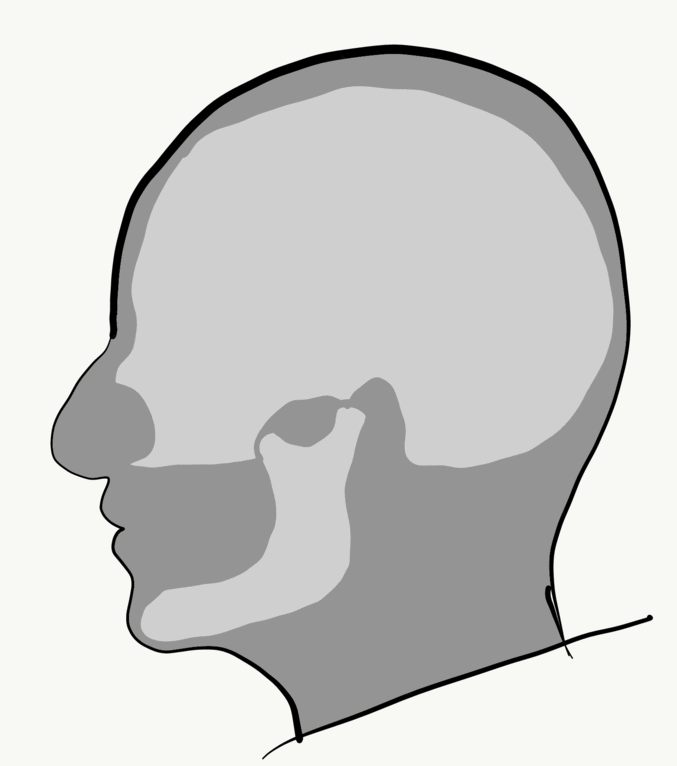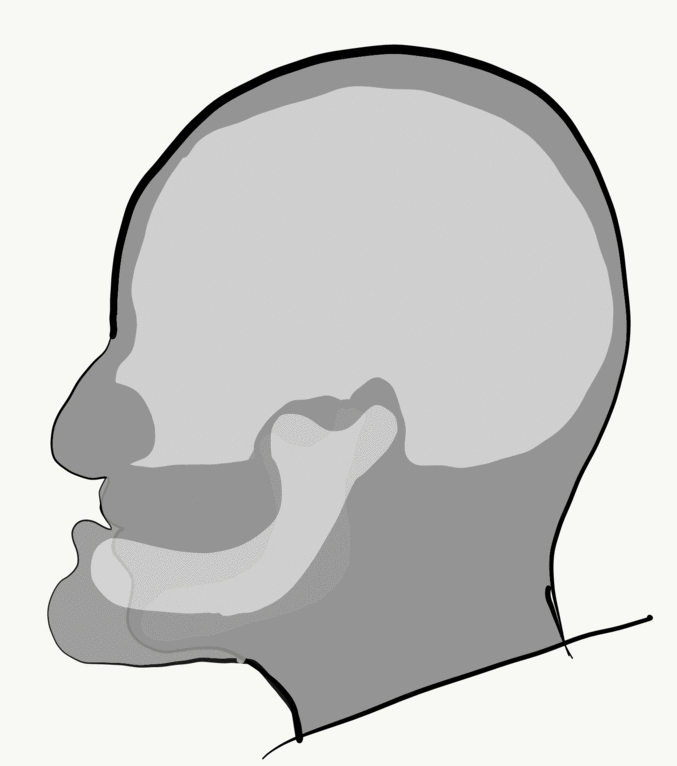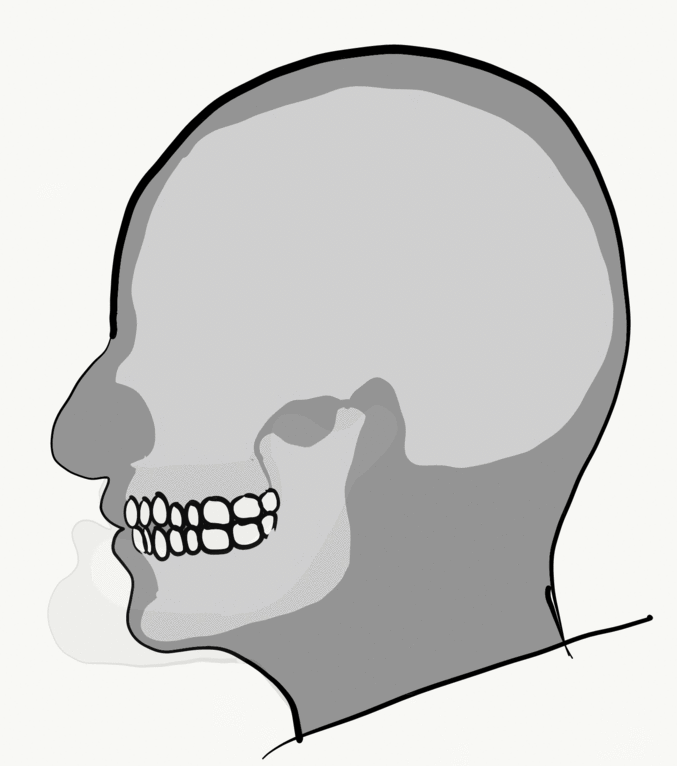
Loss of alveolar bone reduces the vertical dimension of the mandible and maxilla. Without teeth to occlude, the mandible and maxilla can move closer together. This causes the mandible to protrude forward, leading to a “popeye chin”.
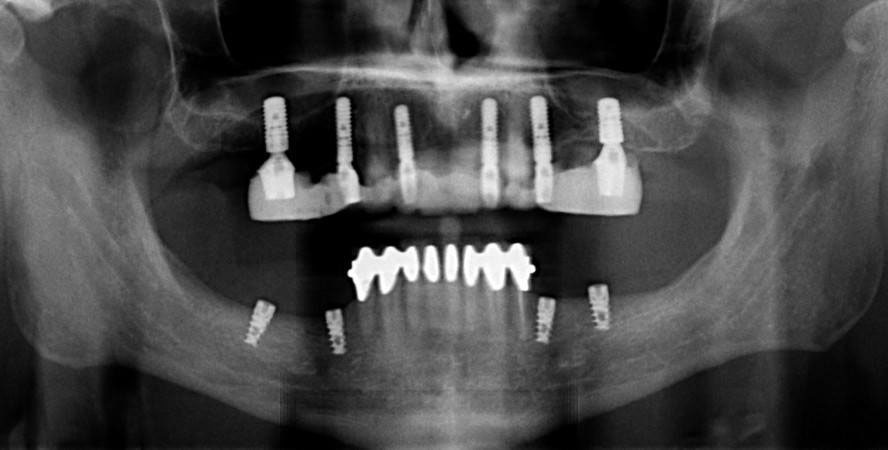
Own work” By Danjhon is licensed under CC BY-SA 4.0Dental implants
Dental implants can prevent loss of alveolar ridge by transmitting force onto bone tissue during mastication. Osteoblasts respond to force by increasing the deposition of calcium and phosphate, increasing bone density and maintaining bone health. If bone loss has already occurred, a bone graft← or Guided Tissue Regeneration procedure may need to be done allow for regeneration of lost bone tissue before the implants are installed. Afterwards, gingival grafting (including SECT←) may be involved if gingival tissue has receded significantly.
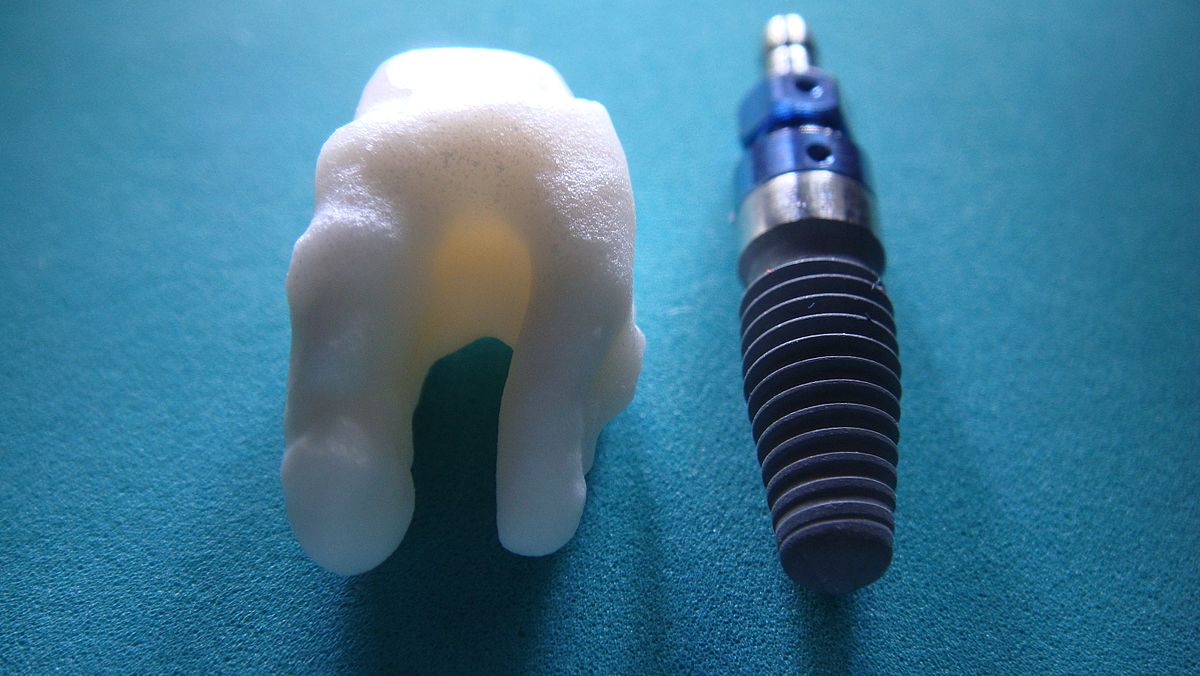
Own work” by Logicwhatelse is licensed under CC BY-SA 4.0Dental Implant technology
Each dental implant consists of a core, abutment and crown. The core is traditionally made of titanium and is implanted into bone tissue, although other technologies exist (Fig. 11.27). To get the shape and size of the core to fit properly within the alveolar ridge involves computed tomography (CT scans) to image bone tissue and computer-aided design (CAD) to specify the core’s dimensions. The abutment connects the core to the crown by a screw or dental cement. The crown is usually made of ceramic. Titanium is strong, durable and bio-compatible. It is more or less invisible to the cell surface receptors that white blood cells use to detect antigens. Unfortunately, this also makes titanium invisible to other cells in the body, including mesenchymal stem cells. As a result, titanium does not adhere well to the connective tissue it is embedded in. Knowledge of histology helps: coating the core with hyaluronic acid improves the connection between the core and the patient’s connective tissue.
Because dental implants fuse to bone tissue, they do not participate in proprioception. There is interest in creating bio-active coatings for the titanium core that might allow PDL attachment to an implant. For instance, layering a core with with hyaluronic acid and PDL stem cells← promotes the connection of a dental implant to PDL in mice.
Another important connection that is lost with tooth extraction is junctional epithelium←. Because the junctional epithelium of each tooth is derived from the REE during tooth eruption←, new junctional epithelium does not form on an implant. At best, oral mucosa may adhere to titanium via hemi-desmosomes←, creating peri-implant mucosa. Coating a ring of the core with morphogens← (such as FGF) mimics the induction of junctional epithlium from REE, improving the connection between oral mucosa and the implant. Coating the titanium core with BMPs, on the other hand, promotes integration with bone tissue.
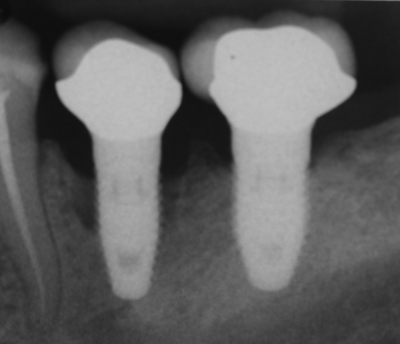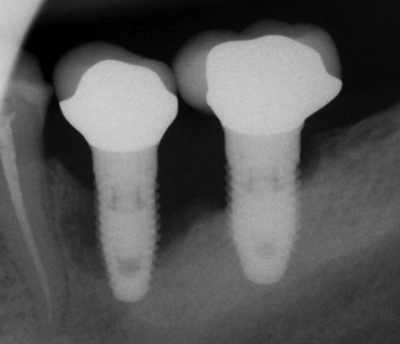
Bone loss following peri-implantitis in a smoker” by Coronation Dental Specialty Group is licensed under CC BY-SA 3.0Peri-implantitis
Failure to create a complete junction between the oral mucosa and the implant exposes underlying connective tissue to the oral microbiome, frequently leading to peri-implantitis. Because the oral microbiome does not go away, chronic inflammation occurs, leading to loss of tissue around the affected implant, including gingival and bone tissue.
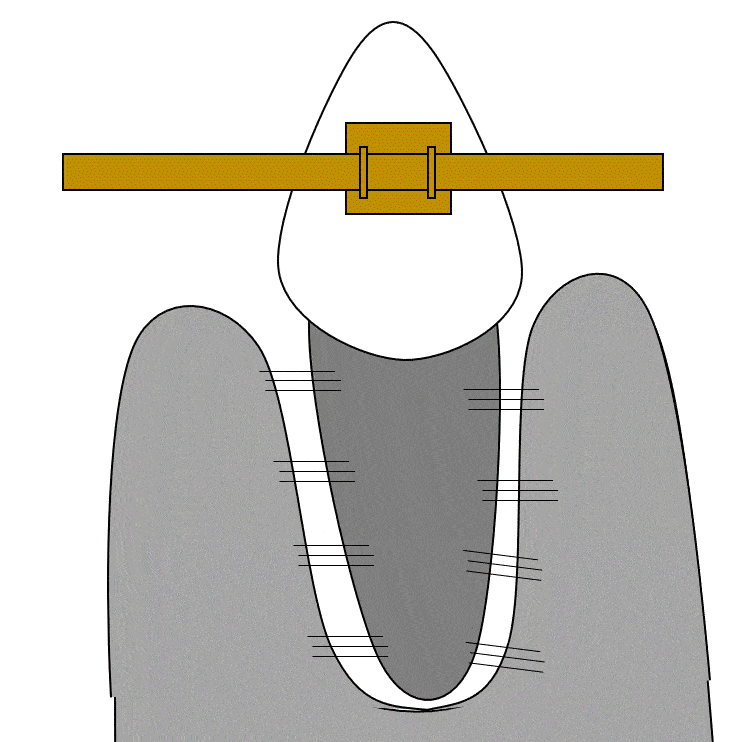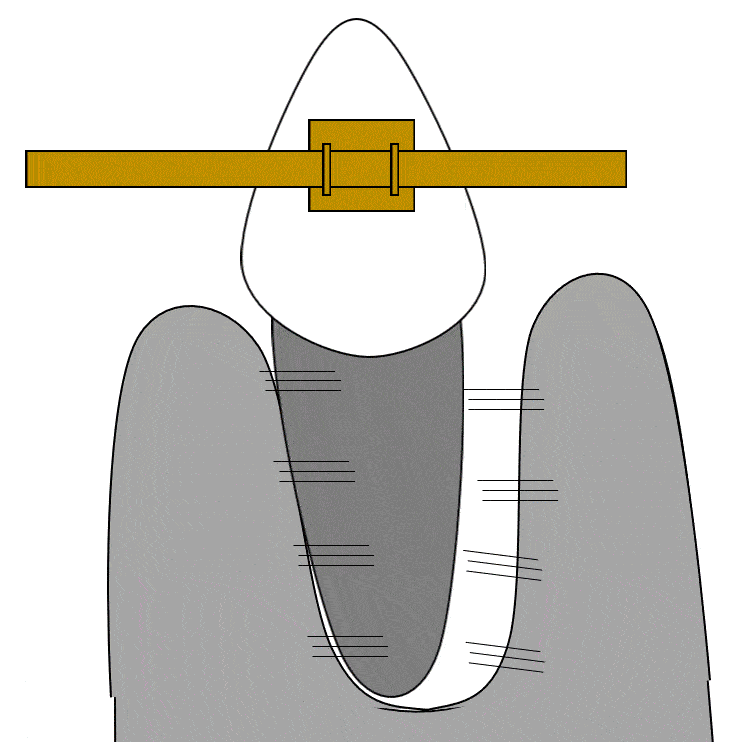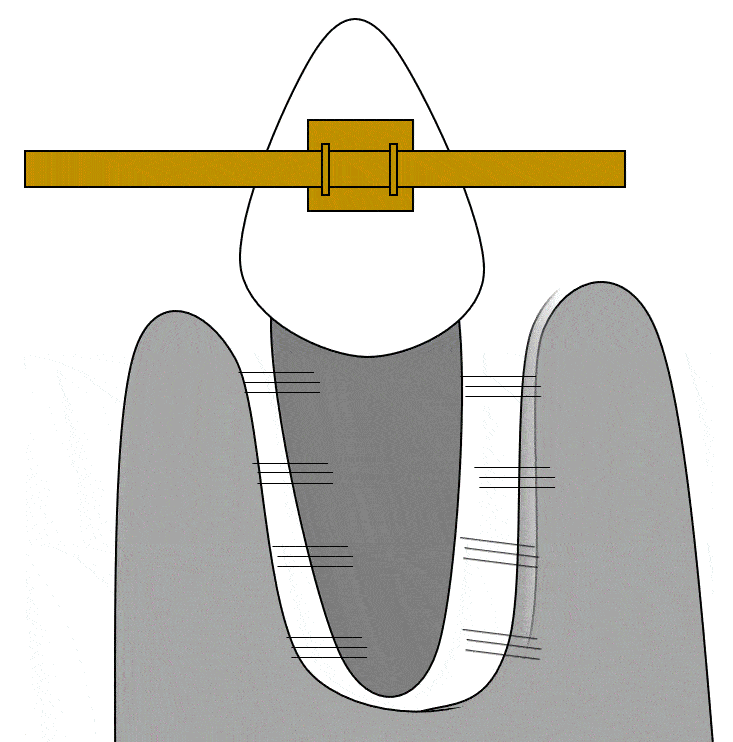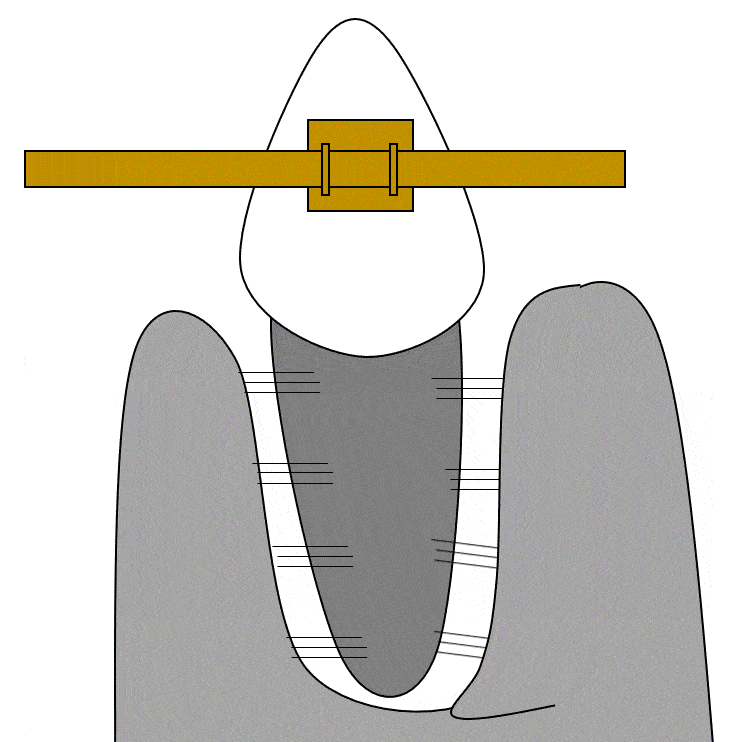
Orthodontia
The remodeling unit← is involved in both maintaining bone health and loss of bone tissue. Its activity is taken advantage of with orthodontia. Pressure applied to a tooth causes the PDL on one side to slacken, and on the other side to tense up. The side with lower amount of tension will undergo bone resorption, as osteoblasts are inhibited but osteoclasts maintain their speed of bone resportion. Higher tension triggers bone deposition on the opposite side. As a result, the location of the alveolar socket shifts in the jawline, without altering the overall dimensions of the socket itself. It is import that the correct amount of force be applied—to much stress can lead to bone resorption all around, increasing the size of the alveolar socket and increasing tooth mobility.
Common treatments for osteoporosis include drugs than inhibit the activity of osteoclasts, such as bisphosphonates. Because the entire remodeling unit is required for orthodontia, patients using these medications cannot undergo orthodontic therapy. Another factor to consider with orthodontia is the activity of odontoclasts and cementoclasts. The morphogens← that induce odontoclast differentiation← and activity (RANKL) during orthodontic movement inhibit the activity of cementoclasts and odontoclasts. For this reason, there is significantly less resorption of cementum and dentin than there is bone tissue during orthodontic movements, even though force across the PDL is the same on bone and tooth root tissues. It is possible to experience root resorption with orthodontic treatment, especially as the orthodontic force increases, although the causes of root resorption are complex and multi-factorial.
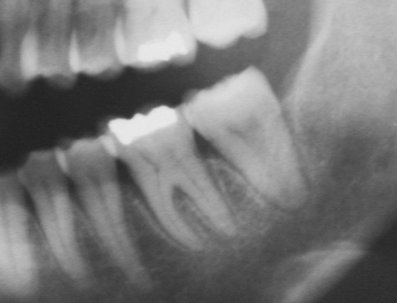
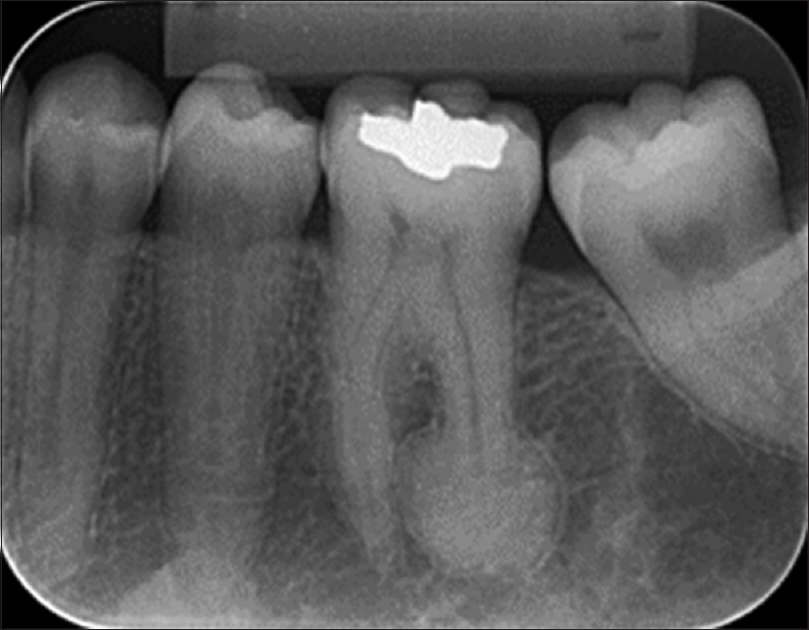
Widening of periodontal ligament space in tooth 36” by CH Chuh is licensed under CC BY-NC-SA 4.0Widening of the PDL space
With occlusal trauma, fibroblasts within the PDL responds with increased activity, leading to a widening of the PDL. For example, in endodontic therapy, a wider PDL space is temporarily generated on the side opposite of the direction of force, but this triggers bone deposition and returns the PDL space to its original width. A wider PDL space can be visualized on a radiograph, and may be accompanied by a thickening of the lamina dura, bone loss, and/or hyper-cementosis. Other conditions besides occlusal trauma may lead to a widening of the PDL space, such as the use of certain medications. The term wider PDL space means that neighboring tissue is lost. It does not necessarily indicate PDL fibroblasts have made more collagen← fibers, only that a larger radiolucent gap exists between the root and the lamina dura. This space may contain more ground substance← or pus, for instance, and not dense regular connective tissue←. This brings us to another example, which may seem contradictory to the first: reduced force can also lead to widening of the PDL space. For example, conditions that negatively affect bone health lead to changes in chewing behavior which in turn reduces occlusal forces. Reduced force is followed by bone loss and a widening of the PDL space. Similarly, chronic inflammation of nearby tissues, such as pulpitis, can trigger widening of the PDL space. Whether these conditions stimulate production of collagen← by fibroblasts is unknown because pulpitis and osteoporosis are associated with increased tooth mobility. Common treatments usually aim to reduce the insult, such as using a nightguard if bruxism is suspected. Dental implants help distribute the forces applied to remaining teeth in a partially edentulous mouth, reducing changes to the PDL space and maintaining tooth attachment.
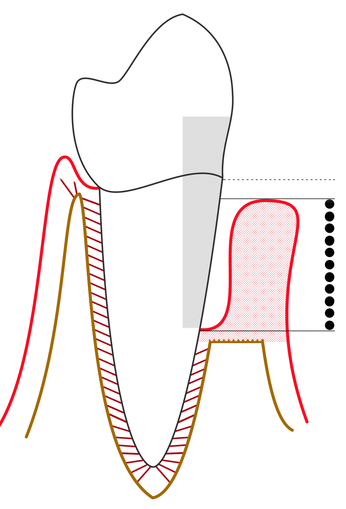
Line diagram of a tooth showing the gingiva, bone, periodontal ligament with a scale showing the pocket depth of severe periodontitis” by Ian Furst is licensed under CC BY-SA 4.0Loss of the PDL apparatus
Loss of attachment between cementum and alveolar bone, known as clinical attachment loss, promotes tooth loss. Two common causes are smoking and bacterial biofilms located within periodontal pockets. With smoking, nicotine plays a role as a toxin. Nicotine activates nicotinic acetylcholine receptors whose activity modulates blood flow, mitosis←, chemotaxis and cell attachment to ECM. These are all necessary for a fibroblast to repair or regenerate the ECM of dense regular connective tissue←. Bacterial biofilms contain toxins which can cause similar changes in fibroblast activity—but these toxins generally only diffuse 1 to 2 mm through ECM, which means biofilms located more than 2 mm away from the DEJ are too far away to harm fibroblasts of the PDL (Fig. 11.31).
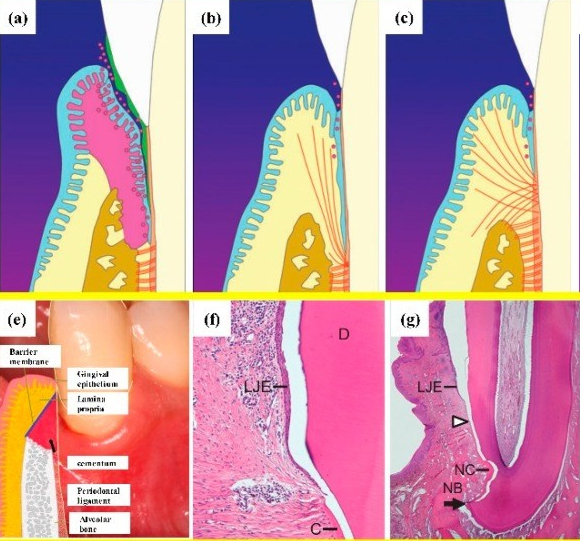
Periodontal regeneration” by Jin Liu, et al is licensed under CC BY 4.0Regeneration of the PDL following periodontitis
We have discussed regeneration of bone tissue using bio-active scaffolds← (guided tissue regeneration), as well as promotion of epithelial attachment to dental implants using ECM protein coatings. The use of similar bioactive membranes to promote regeneration of the PDL has only been studied in animals at this time, not humans. PDL regeneration is conceptually more complicated—which is perhaps the reason why PDL regenerates poorly, and why we have made less progress on PDL regeneration than bone or oral mucosa. The PDL is good at tissure repair because it contains stem cells← and ample vascularity. These two factors that make other connective tissues regenerate well, such as pulp, bone and the sub-mucosa of the oral cavity.
So why is the PDL good at repairing bone and cementum, but not itself? First, the PDL is polarized. It is not enough to stimulate fibroblasts, the cells must be oriented correctly to create the proper attachments (similarly, a good coach won’t tell soccer players to kick the ball into a goal, or even the goal at the north end of the stadium, the target changes at halftime so polarity and time matter). Unfortunately, the planar cell polarity← signals involved in this process are poorly understood. Secondly, the two connections of the PDL form at different times: collagen← fibers are created within pre-cementum during root formation←, but fiber connections to alveolar bone are not made until tooth eruption←. Again, the signals involved are poorly understood, it remains possible that the signals guiding these two connections could be mutually exclusive. We’ve covered examples where the same morphogen← does different things at different times throughout this book. For example, BMP-2 induces the differentiation of odontoblasts, ameloblasts, cementoblasts and osteoblasts. It may not be possible to get the PDL anchored to cementum and alveolar bone at the same time.
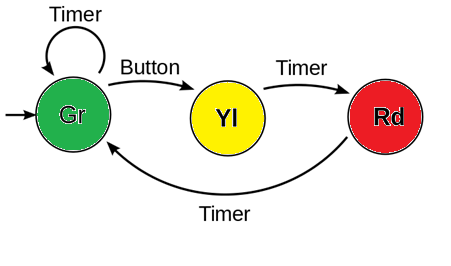
FSA of a traffic light system” by Pluke is in the Public Domain, CC0 / colors addedWithout knowing more specifics, let us offer a metaphor: the two connections of the PDL could be like car traffic at an intersection. For every green light you travel through, cross-traffic has a red light. With proper timing, you might be able to hit green lights all the way down the street, but the traffic light you drove through a minute ago is probably turning red now. It may not be possible to simultaneously get a green light occurring both at the light you are at and the light you were at 5 blocks ago. Urban planners realize that would be a horribly inefficient system. The two ends of the PDL might be controlled like traffic lights, a go signal at one end may be the stop signal at the other end.
Our final thought
Don’t worry about the red light 5 blocks behind you. You have the green light to go apply knowledge of histology and embryology to your clinical coursework. If the light turns yellow, yellow means go faster.
< Chapter 10 * navigation * Chapter 1 >
Chapter review questions
Specialized tissues that surround and support the teeth, including gingival tissue, PDL, cementum and alveolar bone.
Periodontal Ligament, a group of specialized connective tissue fibers that attach a tooth to alveolar bone.
Epithelia that have more than one layer of cells, with the cells at the apical surface having a flat shape.
he most exterior of the three primary germ layers formed in the gastrula, it gives rise to the epithelial tissue covering the body and the CNS.
A series of externally visible anterior tissue bands lying under the early brain that give rise to the structures of the head and neck.
The middle of the three embryonic layers that develop during gastrulaton, mesoderm becomes most of the body's muscle and connective tissues.
Extra-Cellular Matrix: Materials in the body, but outside of cells. A network of macromolecules, such as fibers, enzymes, and glycoproteins, that provide structural and biochemical support to surrounding cells.
The main structural protein in the ECM of connective tissues
Thin protein fibers in the ECM, capable of stretching and returning to their original length, made of the protein elastin.
A subset of mesenchymal stem cells derived from neural crest cells rather than mesoderm, or mesoderm-derived mesenchymal stem cells induced by neural crest morphogens to adopt a more neural fate.
Neuro-mesenchymal cells and fibres surrounding the enamel organ and dental papilla of a developing tooth.
The developmental history of a differentiated cell traced back to the embryonic cell from which it arises.
Elastic-like fibers made of the protein Fibrillin that run parallel to the tooth surface and bend to attach to cementum.
A temporary group of cells that arise from the embryonic ectoderm, and in turn give rise to a diverse cell lineage—including melanocytes, cranio-facial cartilage and bone, teeth and periodontal tissue, smooth muscle, peripheral and enteric neurons and glia.
Hertwig's Epithelial Root Sheath: A proliferation of epithelial cells located at the cervical loop of the enamel organ in a developing tooth which initiates the formation of root dentin.
Programmed cell death
A condensation of ecto-mesenchymal cells seen in histologic sections of a developing tooth.
Inner Enamel Epithelium: a layer of columnar cells located next to the dental papilla, these pre-ameloblast cells will differentiate into Ameloblasts which are responsible for secretion of enamel during tooth development.
The embryonic process in which one group of cells directs the development of another group of cells.
When one cell begins to look different from another. This process involves limiting cell fate by altering gene transcription to become more specialized.
A cell of neural crest origin that is part of the outer surface of the dental pulp, and whose biological function is the formation of dentin.
The formation of dentin.
A group of star-shaped cells located in the center of the enamel organ of a developing tooth, they synthesize glycosaminoglycans.
Shared equally by two parties.
Cells present only during the embryonic period that deposit tooth enamel.
Outer Enamel Epithelium: a layer of cuboidal cells located on the periphery of the enamel organ in a developing tooth.
A substance whose non-uniform distribution governs the pattern of tissue development and pattern formation.
Fibroblast Growth Factors are a family of morphogens involved in a wide variety of processes, including important roles in development and tissue regeneration, especially connective tissues.
Signaling molecules first identified for their role in carcinogenesis, then for their function in embryonic development, including body axis patterning, cell fate specification, cell proliferation and cell migration.
A process by which epithelial cells lose polarity and cell-cell adhesion, and gain migratory and invasive properties to become mesenchymal stem cells, this normally occurs during embryonic development and wound healing.
Differentiated cell that deposits cementum matrix.
Newly formed dentin before calcification and maturation.
Bone Morphogenetic Proteins are a group of signaling molecules, initially discovered for their ability to induce bone formation, now known to play crucial roles in all organ systems.
The process of cementum formation which covers the tooth root by cementoblasts of mesenchymal origin.
The initial un-mineralized form of enamel secreted by ameloblasts.
The thin unmineralized layer present on the surface of developing cementum.
The increase in diameter by the addition of tissue at the surface,
Terminally differentiated cell found within mature cementum lacunae.
Cementum-Dentin Junction
Dentin-Enamel Junction
A layer of dark granules that lie parallel to the outer surface of root dentin.
Bundles of strong type I collagen that anchor the periosteum to bones by penetrating the outer layers of compact bone tissue
Fingerlike projections of the epidermis, the downward-pointing counterparts to the dermal papillae.
Fingerlike projections of the apical side of the dermis, increasing the surface area between the epidermis and dermis, strengthening their connection and increasing exchange of oxygen, nutrients, and waste.
A protein that has had sugar residues attached to it in the Golgi apparatus and is often secreted.
Ca5(PO4)3(OH), the inorganic ECM material composed of calcium, phosphate and hydroxide ions that makes up the bulk of bone tissue.
Cementum containing cells and collagen fibers anchoring the tooth to alveolar bone.
Cementum covering the cervical portion of the tooth root, important for attachment of the periodontal ligament (PDL) to the root surface.
The formation of enamel.
Pain derived from exposed dentin in response to chemical, thermal tactile or osmotic stimuli which cannot be explained as arising from any other dental defect or disease.
A type of connective tissue found in tendons and ligaments that is composed primarily of parallel collagen fibers, secreted by fibroblasts, with very little ground substance.
The basic connective tissue cell type capable of secreting ECM components, including fibers and ground substance proteins.
Lakes, or spaces within cartilage of bone connective tissue where cells reside.
A differentiated connective tissue cell that secretes bone ECM, including collagen, calcium and phosphate.
A cell derived from bone marrow stem cells capable of demineralizing bone tissue by the secretion of acids and enzymes, releasing calcium into the blood.
Undifferentiated or partially differentiated cells that can differentiate into various cell types, and proliferate to produce more of the same stem cell.
Multipotent cells of a connective tissue that can differentiate into a variety of cell types, including osteoblasts, fibroblasts, chondrocytes, myoblasts, blood cells and adipocytes.
Resorptive cell capable of demineralizing cementum by the secretion of acids and enzymes.
Resorptive cell capable of demineralizing dentin by the secretion of acids and enzymes.
Reorganization or renovation of existing tissues, either physiological or pathological. The process can either change the characteristics of a tissue such as in blood vessel remodeling, or result in the dynamic equilibrium of a tissue such as in bone remodeling.
Containing blood vessels
Growth of endothelial cells, creating new blood vessels within a tissue.
Copying DNA into a functional product, such as a RNA and/or protein. Controlled by the activity of transcription factors binding to gene promoter regions to recruit RNA polymerase.
A sequence of nucleotides in DNA that encodes the synthesis of a gene product, either RNA or protein.
Mesenchyme tissue that contains neural crest cell derivatives.
Cell Adhesion Molecules: proteins located on the cell surface involved in binding with other cells or with the extracellular matrix.
Trans-membrane structure specialized for cell-to-cell adhesion.
The process by which animals and plants grow and change.
An endodermal pouch between two pharyngeal arches on the internal surface of the embryo.
A process in tooth development in which the teeth enter the mouth and become visible.
The main principal fiber group is the alveolodental ligament, which consists of five fiber subgroups: alveolar crest, horizontal, oblique, apical, and interradicular on multirooted teeth. Principal fibers other than the alveolodental ligament are the transseptal fibers.
Cemento-Enamel Junction, found in the cervical region of the tooth.
The top (or apex) side of a cell or tissue, usually facing the lumen.
Collagen fibers in the gingiva that, in general, attach the tooth to the gingival tissue (rather than the tooth to the alveolar bone like the PDL fibers).
A polarity axis that organizes cells in the plane (side-to-side) of the tissue.
A very large glycosaminoglycan distributed widely throughout connective tissue ECM, epithelial, and neural tissues.
A large glycoprotein found in the ECM which binds to integrin proteins on the cell surface, involved in cell adhesion, growth, migration, and differentiation.
Clusters of epithelial cells found within the PDL, remnants of Hertwig's Epithelial Root Sheath (HERS).
Formation of bone tissue from a dense connective tissue model
The formation of bone tissue from a cartilage model
Apiece of cartilage from which the mandibles (lower jaws) of vertebrates evolved, originally the lower of two cartilages which supported the first branchial arch in early fish.
A protein that controls the rate of transcription of DNA to mRNA, by binding to a specific DNA sequence.
A group of 235-300 related genes that code for a transcription factors, which control the activity of other genes involved in development, including directing the formation of limbs and organs along the anterior-posterior axis.
An aggregation of cells that eventually forms a tooth, composed of the enamel organ, dental papilla and dental sac.
Small channels in the bone that transmit blood vessels from the periosteum into the bone and that communicate with the haversian canals, or small holes in alveolar bone through which PDL fiber bundles are embedded.
Compact bone that lies adjacent to the periodontal ligament, in the tooth socket.
The bony partition across the alveolar process between adjacent teeth that forms part of the tooth sockets.
The bony septum lying between tooth roots.
The mucous membrane lining the inside of the mouth. It is a stratified squamous epithelium, named the oral epithelium, and an underlying areolar connective tissue named the lamina propria.
The stratified squamous epithelium of the oral mucosa.
The areolar connective tissue layer of the oral mucosa (or hollow organ), homolgous to the papillary layer of the dermis.
The dense irregular connective tissue layer found below the oral mucosa (or mucosa of a hollow organ), homologous to the reticular layer of the dermis.
Epithelium on the inner wall of the gingiva that attaches to connective tissue of the lamina propria on the basolateral side and to the surface of the tooth on the apical side.
Formation and eruption of the teeth.
Reduced Enamel Epithelium: the OEE and ameloblasts found on the surface of the crown prior to tooth eruption.
Idiopathic, non-neoplastic condition characterized by the excessive buildup of normal cementum on the roots of one or more teeth.
A signaling molecule capable of stimulating cell proliferation, wound healing, and cellular differentiation.
A condition of teeth where the cementum overlying the roots of at least two teeth join together.
The exposure in the roots of the teeth caused by a loss of gum tissue and/or retraction of the gingival margin from the crown of the teeth.
A lesion located on the root surface of a tooth, usually close to or below the gingival margin.
The type or types of cell(s) a stem cell can possibly differentiate into in the future, determined by which genes are methylated and stored around histones, or free to be transcribed.
A pulp disease characterized by the loss of dentin as a result of the action of osteoclastic cells stimulated by pulpal inflammation.
Also known as a root canal, is a treatment for infected pulp of a tooth, result in the removal and replacement of pulp and the protection of the decontaminated tooth from future microbial invasion.
When the body's own immune system dissolves the tooth root structure. It can occur following tooth infection, orthodontic treatments or in the presence of unerupted teeth in the jaw.
Abnormal stiffening and immobility of a joint (including tooth-to-alveolar bone)
Spherical calcified bodies lying free in the periodontal membrane.
Materials used in surgical implants, not harmful to living tissue and unlikely to be rejected by the host immune system.
The tendency of teeth to move in a mesial direction within the arch, maintaining interproximal contact between teeth.
Osteoblasts and osteoclasts working together to remove old bone tissue and replace it with new bone tissue, necessary for the maintenance of healthy bones.
Inflammation that damages soft tissue and bone that anchors the teeh, including the gingiva plus PDL, cementum or alveolar bone tissue.
A roughly circular defect of the cortical plate which exposes the underlying root surface, but does not involve the alveolar margin of the bone.
A lack of the facial or lingual alveolar cortical plate resulting in a denuded root surface
A pathologically deepened gingival sulcus around a tooth at the gingival margin.
located at the bottom of the sulcus, consisting of approximately 1 mm of junctional epithelium and 1 mm of gingival fiber attachment.
Having the protein keratin, which lends resistance to abrasion and water loss.
Without blood vessels
Extracellular matrix material involved in the repair of injured and missing tissues, allows stem cells to migrate into the injured area. It is usually replaced during regeneration.
Procedures attempting to regenerate (as opposed to replace) lost periodontal structures.
A medical procedure in which ground up bone tissue or synthetic bone tissue is placed in an injured site to act as a scaffold, promoting the formation of new bone tissue by the patient's own cells.
A strong but immovable connective tissue quickly made by fibroblasts, consisting primarily of highly cross-linked collagen fibers.
A trans-membrane protein which allows cells to bind to the ECM protein fibronectin, it is involved in cell adhesion, growth, migration, and differentiation.
Enzymes that degrade all kinds of extracellular matrix proteins, or process a number of bioactive molecules. They play a major role in cell proliferation, migration, differentiation, angiogenesis, and apoptosis.
An artificial tooth root that is surgically placed into bone tissue.
The use of connective and epithelial tissue from the roof of the mouth or neighboring gingiva, attached to the gum area being treated.
The most common method used to treat root exposure, involving the transplantation of healthy connective tissue from a donor site to the damaged area, which acts a scaffold aiding in tissue regeneration.
A cell-surface, trans-membrane or cytoplasmic protein that binds to (receives) a signaling molecule and transmit the signal further.
A compound that has an effect on a living organism, tissue or cell, such as by mimicking a morphogen or growth factor.
Half of a desmosome, specialized for cell-to-ECM adhesion.
A destructive inflammatory process affecting the soft and hard tissues surrounding dental implants.
The treatment of irregularities in the teeth (especially of alignment and occlusion) and jaws, including the use of braces.
Gel-like substances in the ECM, composed of water held in place by large molecules (proteins, glycoproteins and glycosaminoglycans).
Inflammation of the pulp.
Lacking teeth
Damage to the structures that support the tooth; results from periodontitis and is characterized by relocation of the junctional epithelium to the tooth root, destruction of the fibers of the gingiva, destruction of the periodontal ligament fibers, and loss of alveolar bone support from around the tooth.
The process of cell division, where one cell divides into two identical clones (daughter cells).
After partial loss of as tissue, based on the remaining part, the tissue grows the same structure and function as the lost part.

