3 Histology of the oral mucosa
- Overview
- Skin histology
- Oral mucosa histology:
- Lining mucosa
- Masticatory mucosa
- Dentogingival mucosa
- Specialized mucosa
- Clinical applications:
- Lining & masticatory histology
- Dentogingival histology
- Specialized histology
Overview
We start this chapter reviewing the histology of the skin. The different regions of oral mucosa are similar to the skin because they share the same lineage←: ectoderm and mesoderm. There are a few small differences. Unfortunately, the names we use for the skin are not the same names we use for oral mucosa. Otherwise, it is faster to focus primarily on the small differences, there is no need to cover the histology of the epidermis or dermis each time. In subsequent chapters on the teeth, pay attention to how the lineage of tooth tissues is different from that of oral mucosa, but their basic patterns are similar.
For more practice with histology of the oral mucosa (and more), websites worth visiting are:
The oral histology lab- by Brian R. MacPherson, Ph.D. and James G. Tieman, Ed.D. at the University of Kentucky College of Medicine.
The virtual oral histology lab- By Roger Shore at the School of Dentistry, University of Leeds
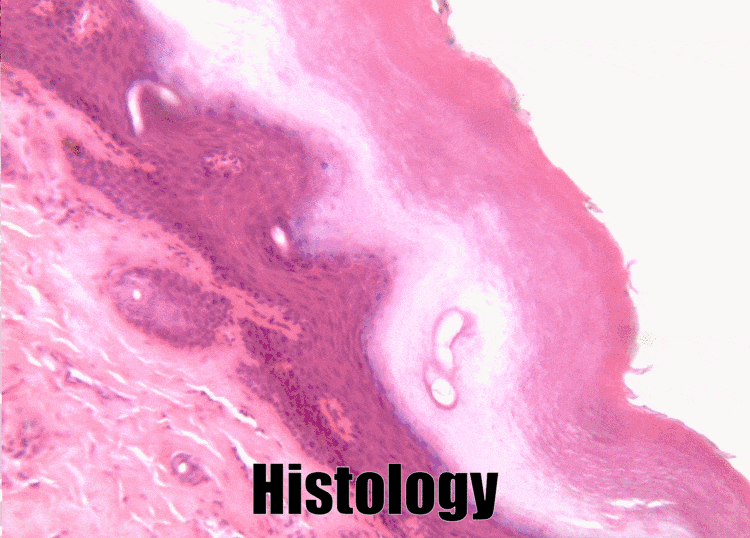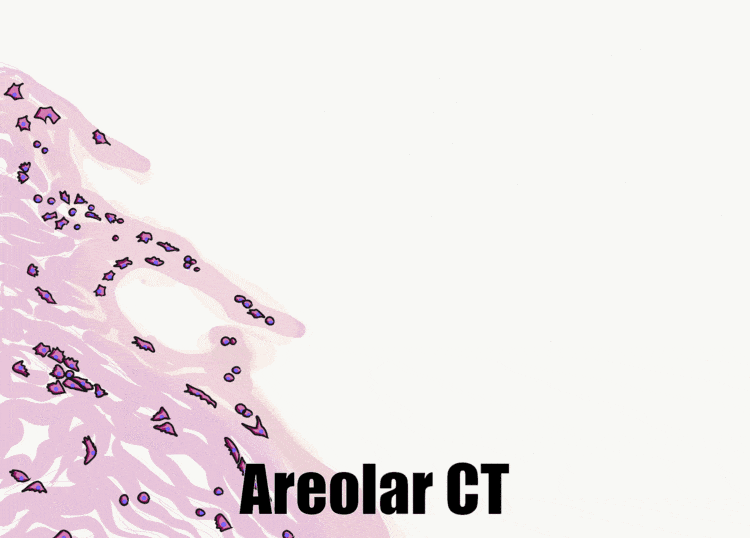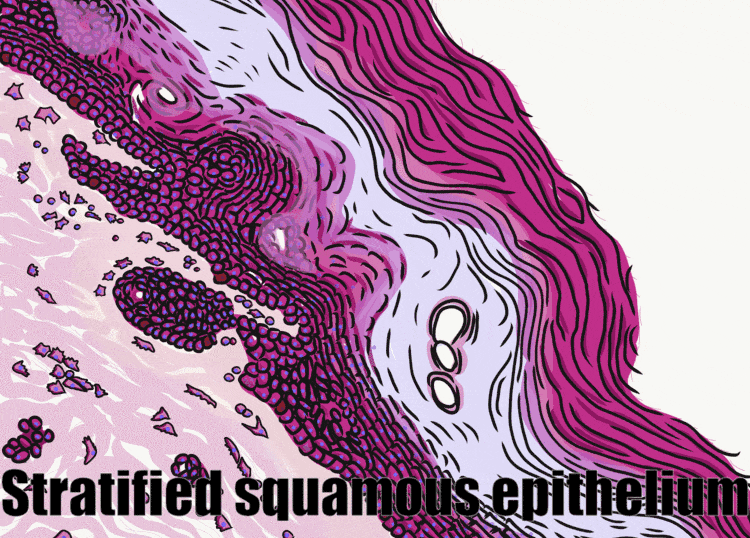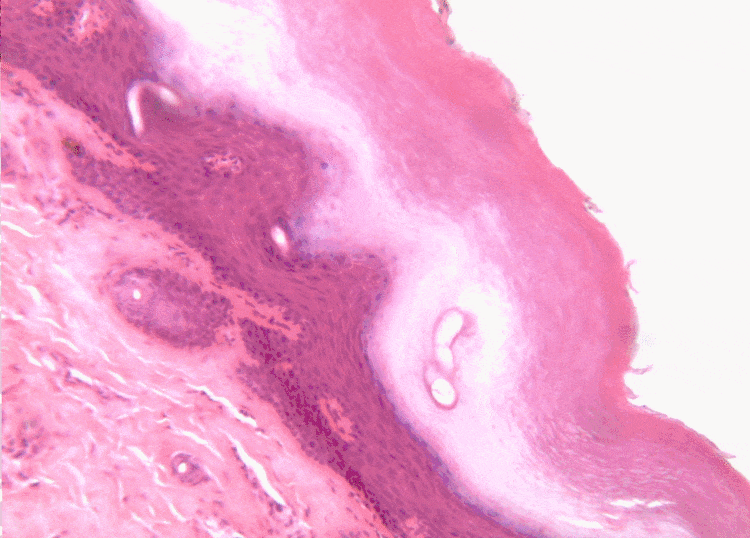
Histology of the skin – for comparison
The skin and the oral mucosa share a lot in common because of their shared lineage← from ectoderm and mesoderm. Both are composed of a stratified squamous epithelium←, just deep to that areolar connective tissue←, followed by dense irregular connective tissue←. Unfortunately, parts of the skin and oral mucosa receive different names and are classified differently based on their location. That means you have more names to memorize… Boo!
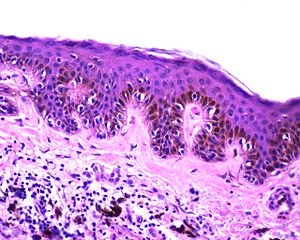
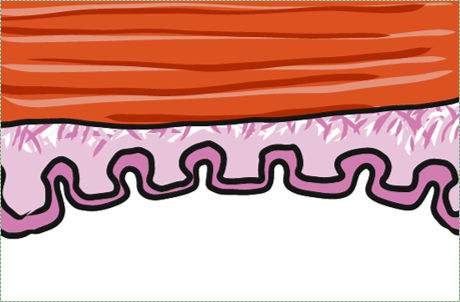
The dermis is the connective tissue of the skin. It is composed in part of a layer of dense irregular connective tissue, the old-fashioned name for which is the reticular layer of the dermis. The other part of the dermis is a layer of areolar connective tissue, which has an old-fashioned name, too: the papillary layer of the dermis. It received that name for having finger-like dermal papillae on the apical surface. In Fig. 3.1 and Fig. 3.7, upward-pointing dermal papillae meet downward-pointing rete pegs of the epidermis. The epidermis is stratified squamous epithelial tissue. The dermal papillae of the dermis meet the rete pegs of the epidermis like inter-meshed fingers from two hands, which makes for a strong connection between epidermis and dermis. Some regions of the oral cavity don’t require such a strong connection, and the rete pegs and dermal papillae are smaller or absent. Note the border between epidermis and dermis is distinct, while the border between the reticular and papillary layers of the dermis is blended. That is because the epidermis is derived from the ectoderm, while the two layers of the dermis are derived from the mesoderm.
The epidermis is highly keratinized. The epithelial cells make a large protein called keratin (or more accurately, keratins, as there are over 50 genes for slightly different keratin molecules). Keratin is similar to collagen←, except keratin is not secreted. Keratins are long fibrous proteins which accumulate within the cytoplasm of keratinocytes, the principle cell of a stratified squamous epithelium←. Epithelial stem cells← in the basal layer of the epidermis give rise to new keratinocytes. As keratinocytes mature, they are pushed towards the apical surface. As the keratinocytes move superficially, they fill up with keratin, and receive fewer nutrients (an epithelium is avascular). Ultimately, the keratinocytes at the surface are dead and completely full of keratin. The keratin fibers are cross-linked to each other and linked to desmosomes←. Desmosomes anchor dead cells together. These links and cross-links make a very tough and water-resistant barrier. The skin is highly keratinized everywhere. Keratinized regions of oral epithelium, on the other hand, are only found in locations where there is a lot of abrasion. In the rest of the oral cavity, moisture is beneficial, and there is less or no keratinization.
Skin color
| Pigment | Color | Source | Location |
|---|---|---|---|
| Melanin | Red or brown/black | Melanocytes | Epidermis/oral mucosa |
| Carotene | Orange-yellow | Diet (plants) | Epidermis/oral mucosa |
| Hemoglobin | Red-maroon | Blood | Dermis/sub-mucosa |
There are 3 major pigments (molecules that absorbs certain frequencies of visible light) that contribute to skin color, listed in Table 3.1. Keratin is not listed because it has no color, but it can obscure the visibility of the deeper pigment hemoglobin. Melanin levels vary naturally in the skin. Everyone is born with roughly the same number of melanocytes, the cells that synthesize the melanin pigment. Melanocytes differentiate← from from neural crest cells← that migrate from the neural tube to the basal layer of the epidermis. The shape of melanocytes more closely resembles neurons or glia than they do epithelial cells. They are not anchored to keratinocytes by desmosomes←, but they attach to keratinocytes by epithelial CAMs←. Attachment to keratinocytes is important to melanocyte function. CAMs allow desmosomes to attach to epithelial cells, but also to let go and migrate away. Melanocytes can produce a lighter form of melanin (pheomelanin) or a darker form (eumelanin). After synthesizing melanin, melanocytes transfer it to keratinocytes inside of vesicles (melanosomes). Because melanocytes migrate, it doesn’t take many melanocytes to distribute melanin to many keratinocytes. The amount and type of melanin produced has a baseline rate set at birth— this is not determined by genes we inherit from our parents, but by more complex epigenetic factors.

"7x speed timelapse video of fish melanophores responding to 200uM adrenaline" by Zephyris is licensed under CC BY-SA 3.0 / converted to gifEnvironmental changes trigger melanocytes to change melanin expression. These changes illustrate several functions of melanin. The most obvious function of melanin is to absorb UV-B light. When UV light causes DNA damage, it induces keratinocytes to secrete a hormone (melanocyte stimulating hormone, MSH). Because the epidermis is avascular, the hormone only travels a short distance. Nearby melanocytes are activated to produce more melanin. Extra melanin, when deposited into new keratinocytes, reduces damage to DNA and helps prevent skin cancer. Tan lines illustrate just how far MSH diffuses and melanocytes migrate– not very far on on the scale of the human body.
Melanin (in the epidermis) also protects folic acid levels in the blood (the dermis) from UV light exposure. For light-skinned people, one hour of sun exposure destroys about half of their folic acid. This is significant because folic acid is required for cell division. Low levels of folic acid during pregnancy lead to congenital malformations of the neural tube← (spina bifida).
"Lentigo simplex or simple lentigo or lentiginous melanocytic naevus“ by Leszek Woźniak & Krzysztof W. Zieliński is licensed under CC BY-SA 3.0Melanin may be present within the oral cavity, despite very low levels of UV light exposure. This can be explained because there are two more important functions of the molecule melanin. Melanin protects tissues against abrasion. Pregnancy leads to an increase in the amount of melanin in the areola and labia minora, areas likely to suffer more abrasion during or after childbirth, not more UV damage. A third function of melanin is its ability to bind free radicals. This function requires a team effort between melanocytes and keratinocytes. Melanocytes make and transfer melanin to keratinocytes, where it binds toxic free radicals (often caused by UV damage). That alone would not protect the skin, it would trap free radicals in keratinocytes. But as keratinocytes are exfoliated, melanin helps remove free radicals from the body.
Two other major skin pigments are hemoglobin and carotene. Higher levels of the darker eumelanin may hide them. Hemoglobin allows red blood cells to pick up and dump off oxygen molecules. It undergoes a color change when it picks up oxygen, shifting from maroon to red. Healthy people of any skin color do not exhibit variation in hemoglobin. Keratin, however, can make skin appear less reddish. Because hemoglobin is in the dermis and keratin is in the epidermis, high levels of keratin hide hemoglobin.
Carotene is an orange pigment molecule made by plants. Unlike melanin, carotene is not made in the human body. We ingest carotene from our diet, and from there it accumulates in the epidermis and oral epithelium. Carotene is converted into Vitamin A, which is converted into the important morphogen← retinoic acid. Vitamin A is also a necessary co-factor in the methylation and histone modification of DNA. The amount of carotene that accumulates in the skin, along with melanin, contributes to diversity in skin tone. Unlike melanin, skin carotene levels usually do not change in response to environmental factors. The genetic or epigenetic factors that contribute to heritable skin carotene levels (yellow skin tones) are poorly understood.

"Anatomical features of the lips of a baby" by Occlusion is licensed under CC BY-SA 4.0Vermilion zone
The vermilion zone (or red zone of the lips) is sometimes defined as part of the skin, or other times defined as part of the oral mucosa. It contains less keratin, no hair follicles and few if any sebaceous glands. Furthermore, its keratinocytes synthesize a clear protein (eleidin). This allows the reddish color of hemoglobin in the capillaries of the dermis and muscle tissue to be more visible than in more highly keratinized regions of neighboring skin. After you finish reading about oral mucosa, decide for yourself whether the vermilion zone should be considered a part of the skin, oral mucosa, its own tissue, or create your own way of classifying the three.

Hair follicles
Hair follicles share developmental processes with teeth. That gives these two appendages of the skin and oral mucosa a similar pattern, only one makes keratin and the other calcium hydroxyapatite. A hair follicle is an invagination (an inward-folding of a tissue, such as during neurulation←) of the epidermis. The stem cells← in the basal layer of the hair bulb divide, differentiate← into keratinocytes, and die to form the hair itself. A hair, therefore, is an epithelial structure. Surrounding– or deep to– the hair follicle are the connective tissue layers of the dermis.
Where and when hair follicles invaginate from the surface epidermis is regulated by planar cell polarity← signals. These signals ensure roughly-even spacing between follicles. The same signals govern the spacing of the teeth. Also similar to teeth, new hair follicles grow beneath old ones, pushing the old ones out in a process called exfoliation. Of course, hair follicles grow and exfoliate more times than a tooth. Nevertheless, it might be a good idea to keep scientific developments in the hair-loss treatment industry somewhere on your radar, as advances there may have applications in some future tooth-growth industry.
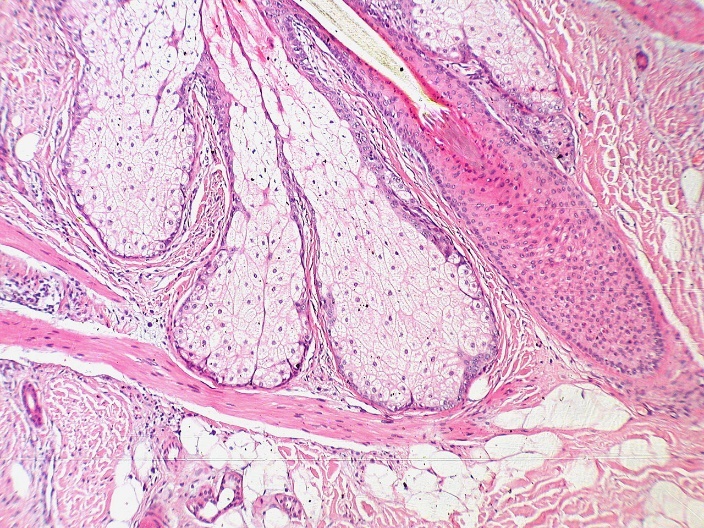
"Base of pilosebaceous unit" by Kilbad is in the Public Domain, CC0Sebaceous glands
Sebaceous glands are oil-producing glands within the skin. They may discharge directly to the surface of the skin, but are mostly associated with hair follicles. The material they secrete, called sebum, is mostly lipids (triglyceride and others). Unlike proteins, these molecules are not synthesized on the rER←, modified in the Golgi apparatus← nor secreted from vesicles. Instead, sebum accumulates in the cytoplasm and is secreted by destruction of the cell. New glandular cells are produced from stem cells← in the basal portion of the gland. The vermilion zone and oral mucosa contain few or no sebaceous glands, but when sebaceous glands are present in these regions, they form benign whitish spots known as Fordyce Spots.
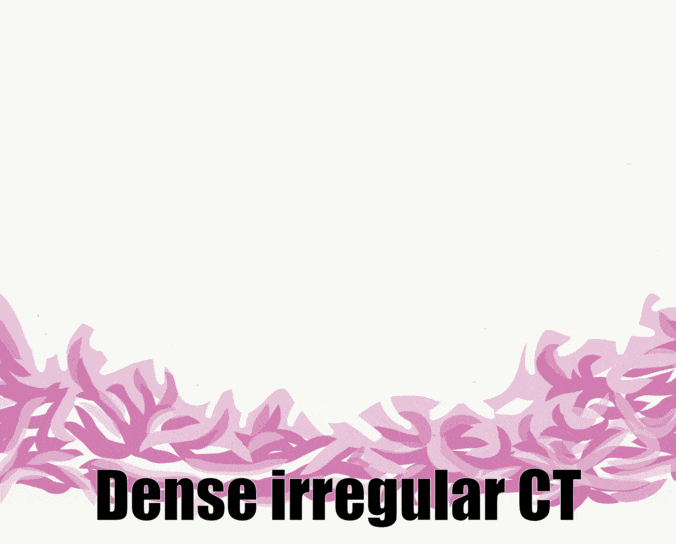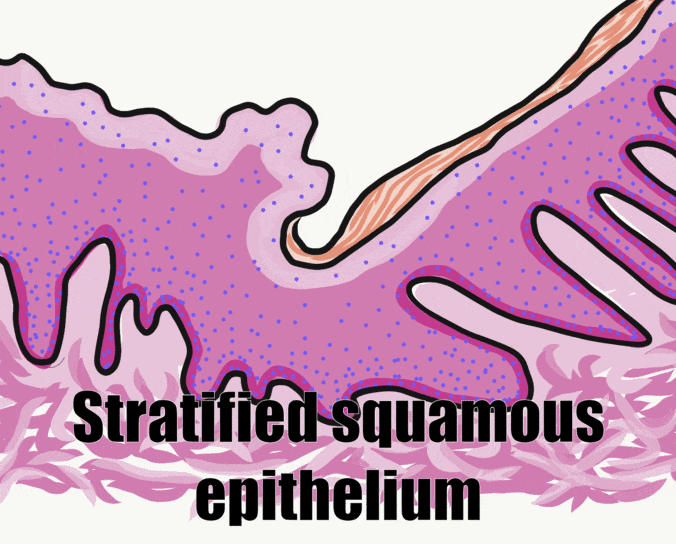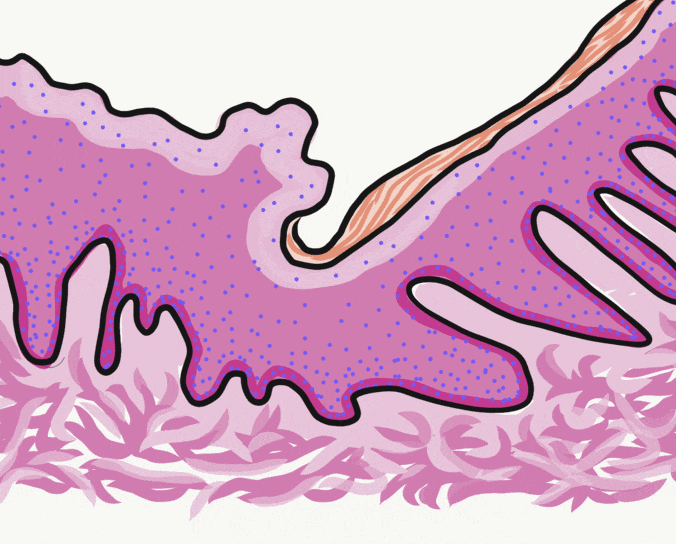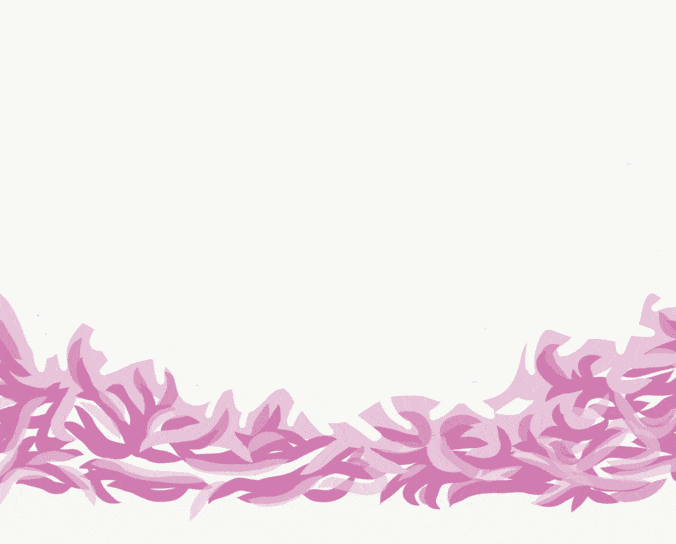
General histology of the oral mucosa
The oral mucosa is the mucous membrane that lines the oral cavity. It shares the same lineage← as the skin, and we therefore see the same tissue types in the same order. However, its layers get different names and are classified differently. Based on the 3 embryonic germ layers, the layers of the skin are divided appropriately, but the oral mucosa is divided incorrectly.
First, stratified squamous epithelium← and underlying areolar connective tissue← are lumped together and called the oral mucosa. The stratified squamous epithelium may be referred to individually as the oral epithelium, and like the epidermis, is derived from the ectoderm. The layer of areolar connective tissue←, which is homologous to the papillary layer of the dermis, is called the lamina propria. It is produced by cells derived from mesoderm. Deep to the oral mucosa, the layer of dense irregular connective tissue← is called the sub-mucosa, and is the homologue of the reticular layer of the dermis. The sub-mucosa is also produced by cells derived from mesoderm.
| Embryonic tissue | Skin | Oral mucosa | ||
|---|---|---|---|---|
| Ectoderm | Epidermis | Stratified squamous epithelium | Stratified squamous epithelium | Oral mucosa |
| Mesoderm | Dermis | Areolar CT | Areolar CT | |
| Dense irregular CT | Dense irregular CT | Sub-mucosa | ||
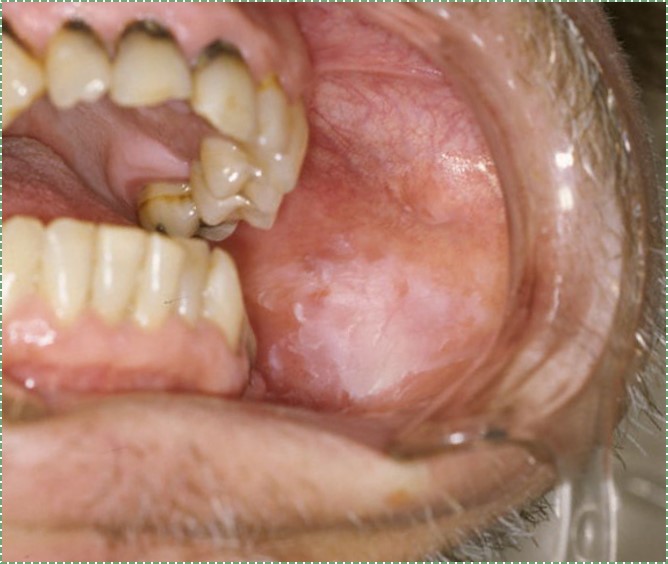
leukoplakia by dozenist is licensed CC BY 3.0The amount of keratinization of the oral mucosa reflects the amount of stress or abrasion that region experiences. This is similar to the formation of a callus on the hands or feet. Higher-than-normal levels of keratinization are clinically relevant when they indicate bruxism, tobacco use, or other health-related issues. Keratin doesn’t have a color, but higher levels of keratin in the epithelium obscures the maroon color of blood found within the lamina propria and sub-mucosa, hence non-keratinized mucosa look more reddish, while keratinized mucosa more whitish. Levels of keratinization are categorized into three or four groups listed in Table 3.3.
| Type of stratified squamous epithelium | Level of keratinization | Location |
|---|---|---|
| Keratinized | Full | Skin |
| Ortho-keratinized | Partial | Masticatory mucosa |
| Para-keratinized | ||
| Non-keratinized | None | Lining mucosa |
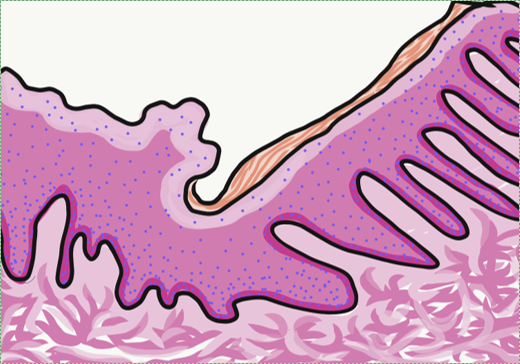
3 classes oral mucosa
Lining mucosa is found in most regions of the oral cavity, and is not involved significantly with mastication. These are regions more important for speech and swallowing. They are therefore mostly non-keratinized. They may have higher levels of elastic fibers← within the lamina propria. Because lining mucosa does not get as much friction and abrasion, it has small or no visible dermal papillae and rete pegs between the epithelium and connective tissue layers. The left-half of Fig. 3.9 illustrates a lining mucosa.
Masticatory mucosa is found in regions of high abrasion caused by mastication, such as the attached gingiva. The epithelium is either be ortho-keratinized or para-keratinized, which are both partially keratinized. An ortho-keratinized epithelium contains keratinocytes with keratin and nucleuses, whereas the para-keratinized epithelium lacks nucleuses. Differentiating between ortho- and para-keratinized tissue is based on appearance, and has no clinical significance. For the rest of this book, we refer to them together as “ortho- or para-keratinized epithelium”. Because this mucosa is generally under higher levels of stress, it has more pronounced dermal papillae and rete pegs than lining mucosa. The right-half of Fig. 3.9 illustrates a masticatory mucosa, whose apical surface contains a degree of keratinization. Where a lining mucosa and masticatory mucosa meet is a border referred to as the mucogingival junction.
Specialized mucosa is found on the dorsal surface of the tongue. More important than its level of keratinization is the precense of specialized structures, such as lingual papillae and taste buds.
Lining mucosa
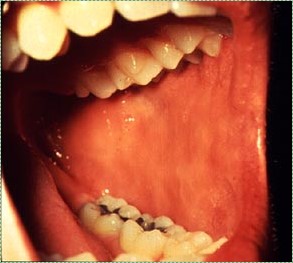
buccal mucosa" by the NIH is in the Public Domain CC 0Labial and buccal mucosa
Labial mucosa and buccal mucosa both have a non-keratinized stratified squamous epithelial← layer. This gives them a more reddish or pinkish base appearance (see alveolar mucosa pigmentation below). As with all oral mucosa, there are no hair follicles, but in places sebaceous glands may be present, forming Fordyce spots. As a lining mucosa, the epithelial layer is generally non-keratinized, but there can be regions of keratinization where stress occurs. Most likely, the lnea alba (or “white line”) will be apparent, running along the line in the buccal mucosa where the teeth meet.
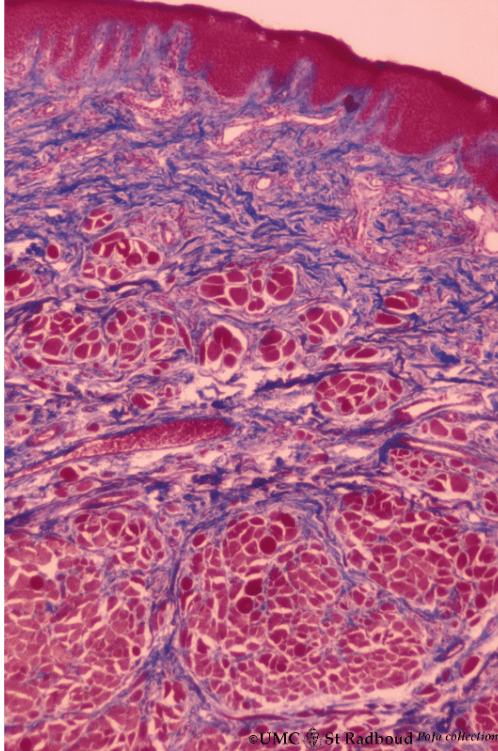
"Lip (human), region between red zone (vermilion border) and mucosa inner surface" by Poels, Lambert G. is licensed under CC BY-NC-ND 3.0Fig. 3.11 shows the histology of where the vermilion zone and labial mucosa meet. More prominent rete pegs and dermal papillae of the labial mucosa compared to the vermilion zone develop in response to higher levels of abrasion (nurture), and do not represent a lineage← difference between these two tissues (nature).
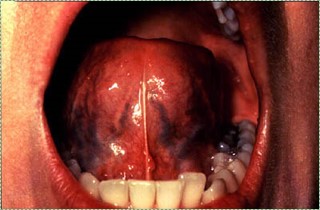
Ventral tongue by the NIH is in the Public Domain CC 0Ventral surface of the tongue and the floor of the mouth
The ventral surface of the tongue and the floor of the mouth both contain very thin, non-keratinized stratified squamous epithelium←. The thinness gives these surfaces a more reddish-appearance than other lining mucosa. The thinness of the epithelium, coupled with the rich blood supply in the deeper lamina propria are also why some medications are given sublingually.
Soft palate
The soft palate is lined by a non-keratinized stratified squamous epithelium←, with a very thin layer of sub-mucosa deep to it. This gives the epithelium a firm attachment to deeper muscle tissue, which is important for speech and swallowing.
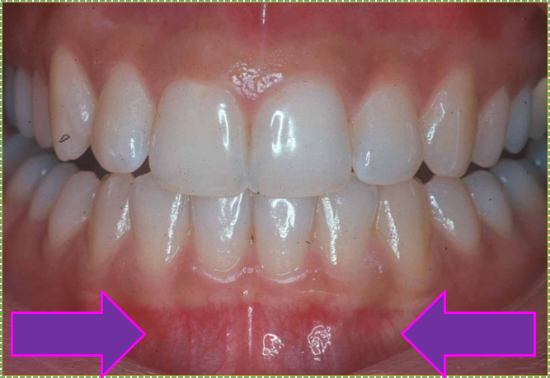
"Gingiva of the human mouth” by John Crawford is licensed CC BY 3.0. / Arrows addedAlveolar mucosa
Alveolar mucosa is lined by a non-keratinized stratified squamous epithelium←. It has a rich blood supply and numerous elastic fibers← within the lamina propria, but few dermal papillae and rete pegs. Because of the large blood supply and low levels of keratin, you may often hear healthy alveolar mucosa should appear pinkish, but this assumes the absence of melanin.
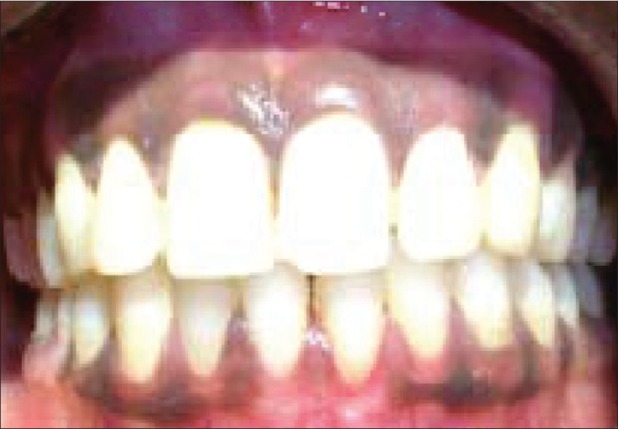
"Preoperative picture of 25-year-old female complaining of black-colored gums" by Arthiie Thangavelu et al is licensed under CC BY-NC-SA 4.0Clinical disorders may alter the coloration of the gingiva, but to assume all healthy gingiva are pinkish ignores healthy variation in skin color. The gingiva of darker-skinned patients may be darker and be completely healthy. Melanin can be deposited in alveolar mucosa, either uniformly (evenly) or focally (localized, such as a freckle or macule, a freckle on the lips). Tanning is a homeostatic change in melanocyte activity in response to UV damage to keratinocytes (nurture). The presence of melanin in the gingiva, however, rarely represents a homeostatic change (hence, not a pathological change). Instead, melanin levels in the oral mucosa are linked to inborn melanin level in the skin (nature), a concept that should be more obvious once we consider the shared lineage← of these two tissues.
Masticatory mucosa
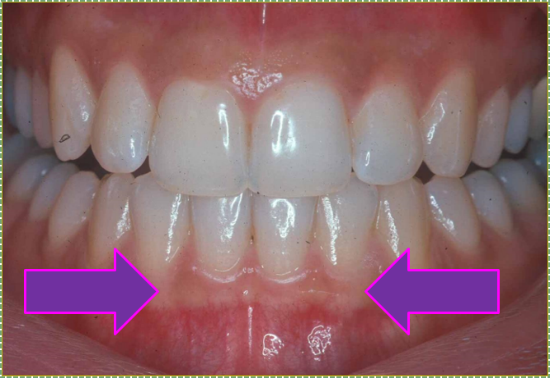
"Gingiva of the human mouth” by John Crawford is licensed CC BY 3.0 / arrows addedAttached gingiva
Attached gingiva is a type of masticatory mucosa, lined with a para-keratinized stratified squamous epithelium←. The increased amount of keratin, compared to alveolar mucosa, obscures the underlying blood supply, creating a lighter appearance (which can be described as whitish in the absence of melanin). The attached gingiva is named for its firm attachment to the tooth or to alveolar bone by groups of gingival fibers.
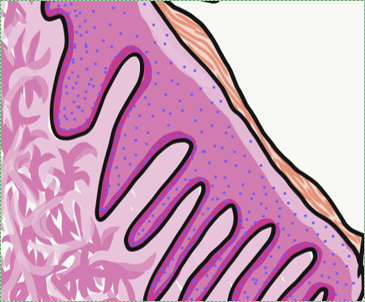
Large dermal papillae and rete pegs create the stippled (rough surface) appearance of the attached gingiva. The rough surface may also be described as orange-peel, which indicates the relative health of the attached gingiva due to sizeable rete pegs and dermal papillae between the oral epithelium and lamina propria.
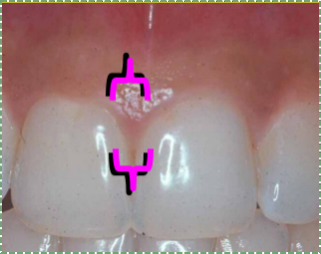
"Gingiva of the human mouth” by John Crawford is licensed CC BY 3.0 / cropped and brackets addedInterdental gingiva
Interdental gingiva (or the interdental papilla) is similar to the attached gingiva.
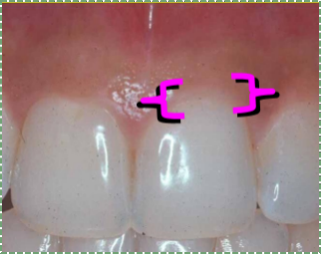
Marginal gingiva
Marginal gingiva epithelium is histologically similar to the attached gingiva— it has pronounced rete pegs and is partially keratinized. The gingival margin may be grouped together with junctional epithelium and sulcular epithelium (described below) and referred to as free gingiva. Unlike the attached gingiva, the sub-mucosa of free gingiva is not connected to bone tissue.
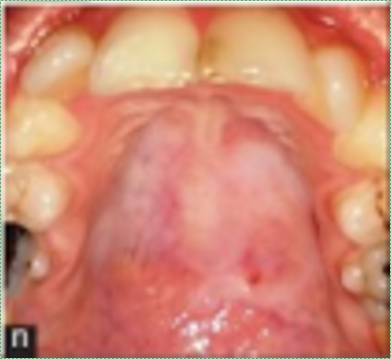
Pleomorphic adenoma of the left palate” by the NIH is in the Public Domain CC0Hard palate
The hard palate is lined by an ortho-keratinized stratified squamous epithelium← and mostly lacks a sub-mucosa, making for a rigid connection to underlying bone tissue←.
Mucosa of the Dento-Gingival junction
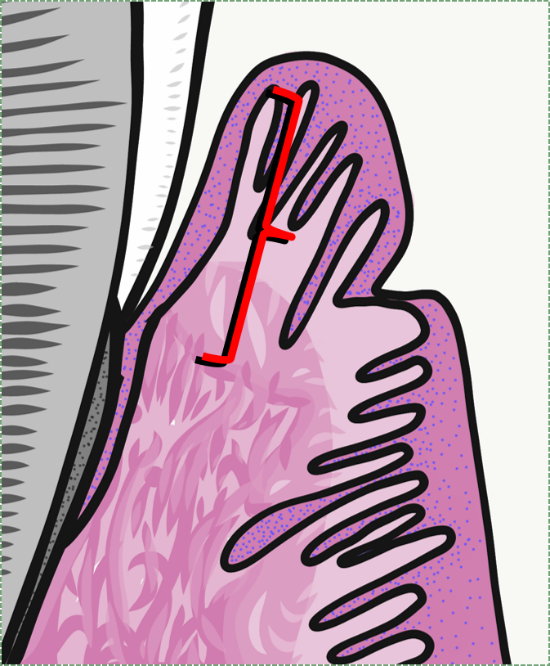
Sulcular epithelium
Sulcular epithelium (or crevicular epithelium) is lined by either a non-keratinized or para-keratinized stratified squamous epithelium←. It creates a space between the gingiva and tooth, named the gingival sulcus. Sulcular epithelium is not attached to the surface of the tooth. Under the microscope, the absence of dermal papillae and rete pegs indicates this tissue gets very little abrasion under healthy conditions. It is also more delicate and permeable, especially in the deeper regions closer to the junctional epithelium.
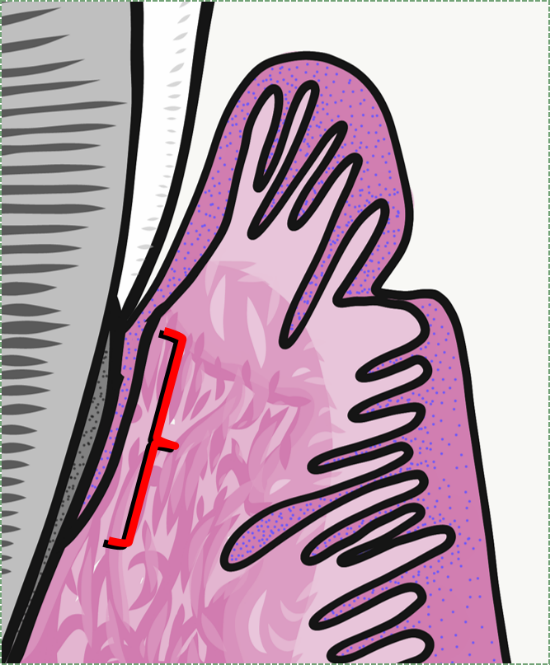
Junctional epithelium
Junctional epithelium (JE) is a non-keratinized stratified squamous epithelium←. It is special in that its apical surface attaches to the tooth by way of hemi-desmosomes←. Other epithelia attach to connective tissue only on their basolateral surface, their apical surface faces the external environment. This unique attachment to the tooth surface is referred to as the epithelial attachment (EA). This uniqueness is challenging to re-create with dental implants. In a subsequent chapter, we cover the lineage← of the junctional epithelium that gives the adult tissue its uniqueness.
Junctional epithelium is thinner than other gingival mucosa, only five cells wide at the end (relative to the apical-to-basolateral direction of the epithelium, not the apical-to-coronal direction of the tooth). It is more permeable, having fewer desmosomes← between cells. This allows white blood cells from the underlying vascular sub-mucosa to migrate through junctional epithelium and enter the gingival sulcus. But this also increases the potential for oral cavity bacteria to do the same in reverse, especially if the epithelial attachment is lost. This is an important factor in the study of periodontal infection and the immune response.
Specialized mucosa

Tongue histology
The dorsal surface of the tongue contains multiple types of mucosa. This should make moe sense after we cover how the tongue develops from three different pharyngeal arches←. The epithelial surface is mostly an ortho-keratinized stratified squamous epithelium←, and can therefore be thought of as a masticatory mucosa. Scattered across the dorsal and lateral surfaces are four different shapes of bumps called lingual papillae. The most numerous are filiform papillae, which contain only keratinocytes, and may be ortho- or para keratinized. The other three lingual papillae also contain taste buds, which are not keratinocytes, and therefore not a masticatory or lining mucosa, but a specialized mucosa. These structures are appendages of the oral mucosa.
Anteriorly, deep to the oral mucosa and sub-mucosa, the tongue contains numerous bundles of skeletal muscle cells, and some adipose tissue. Posteriorly, the tongue contains more adipose and salivary gland tissue, and is covered by tonsillar tissue rather than an ortho-keratinized stratified squamous epithelium.
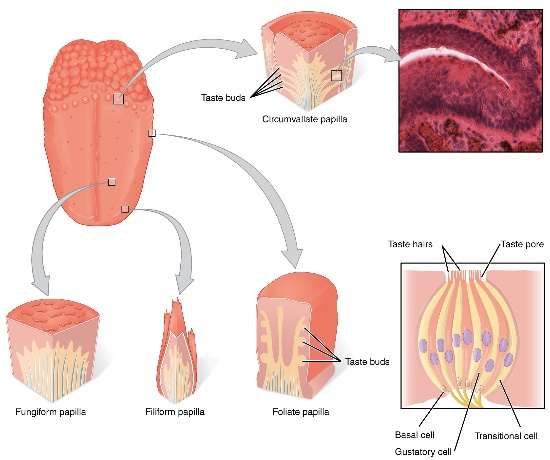
"The Tongue” by the OpenStax is licensed CC BY 4.0Filiform papillae are the majority of the tongue’s dorsal surface, giving it avelvety appearance. They contain an ortho-keratinized or para-keratinized stratified squamous epithelium←. These papillae function to provide friction only, their mucosa contain no taste buds.
Fungiform papillae are shaped like a mushroom (a type of fungus) and are dotted throughouot the dorsal surface of the tongue. They contain an ortho-keratinized or para-keratinized stratified squamous epithelium← over a highly vascular sub-mucosa, giving these structures a more reddish-appearance than neighboring filiform papillae. The epithelial layer contains taste buds, which detect the sense of gustation, which is in turn a part of the perception of taste.
Foliate papillae are found on the lateral edges of the tongue. They contain an ortho-keratinized or para-keratinized stratified squamous epithelium← with taste buds.
Circumvallate papillae are found in a V-formation at the border between the anterior and posterior portion of the tongue, the sulcus terminalis. They contain an ortho-keratinized or para-keratinized stratified squamous epithelium← with taste buds and minor salivary glands.
Turnover time of epithelia
| Epithelium | Turnover time (days) |
|---|---|
| Skin | 27-38 |
| Hard palate | 24 |
| Floor of mouth | 20 |
| Buccal and labial mucosa | 14 |
| Attached gingiva and taste buds | 10 |
| Junctional epithelium | 5 |
The time it takes to replace all of the cells within the epithelial layers of the skin and oral mucosa is shown in Table 3.4. As you should see, the oral epithelium grows quickly, which means it can regenerate quickly following injury. This is largely due to the presence of growth factors in saliva. This also means the lifespan of these cells is short, making oral cancers relatively rare in the absence of large doses of carcinogens (tobacco and alcohol). The epithelial cells of oral mucosa do not live long enough to easily acquire the multiple mutations to oncogenes and tumor-suppressor genes required to cause cancer.
Clinical applications
Hyper-keratosis
Hyper-keratosis is a homeostatic response of the oral mucosa to stress, either chemical or physical (e.g. smoking or denture friction). In response to stress, epithelial cells express more keratin, causing an increase in the degree of keratinization. Vitamin A-deficiency can lead to generalized hyper-keratosis.
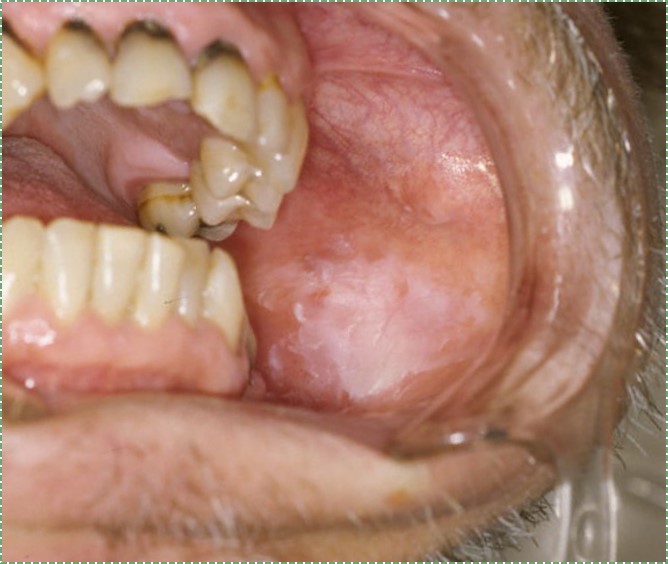
leukoplakia" by dozenist is licensed CC BY 3.0Leukoplakia
If the increase in keratinization is localized, it is referred to as leukoplakia. Parafunctional habits can cause regions of the buccal mucosa to undergo hyper-keratosis. Bruxism may cause the linea alba to appear more whiteish. Chemical stress caused by use of smokeless tobacco products (snuff) cause leukoplakia at the site of use.
Beta-carotene supplements may be prescribed in the treatment of leukoplakia because carotene accumulates within the oral epithelium (and epidermis). There, it is converted into Vitamin A, which in turn is used to synthesize the morphogen← Retinoic Acid (RA). RA induces the differentiation← of epithelial stem cells← into keratinocytes rather than undergoing mitosis←. After a week, this leads to fewer keratinocytes, reversing the severity of lesions caused by over-production of keratinocytes (psoriasis in skin) and keratin (leukoplakia in oral mucosa). However, beta-carotene supplementation is not effective at preventing the progession of leukoplakia into oral cancer in smokers.
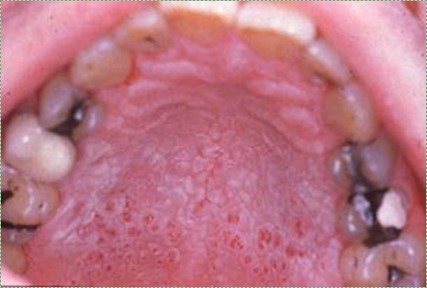
"Nicotinic stomatitis" by DVIDS is in the Public Domain CC0Nicotinic stomatitis
Hyper-keratosis can be caused by the chemical stress of cigarette smoke. It is not caused by nicotine, which is an addictive substance but mostly non-toxic to adult humans, despite what many otherwise reliable resources suggest. Nicotine is a teratogen to developing embryos. We cover adverse effects of nicotine with PDL loss in chapter 11. For the oral mucosa (and respiratory tract), the chemical stress from smoking is caused by benzene, dioxin, formaldehyde, poly-aromatic hydrocarbons, and other toxic chemicals produced by combustion (burning), not the tobacco plant. At the time we are writing this, there is no evidence that nicotine gum causes hyper-keratosis of the oral mucosa, and the link between vaping and hyper-keratosis is not strong, despite often containing nicotine. If your patients smoke, these two nicotine-delivery methods are considerably safer for both the lungs and oral cavity, and can be helpful tools in smoking-cessation (nicotine is the addictive component of tobacco products). This is not to be mistaken for an endorsement of taking up an e-cigarette habit, nor maintaining one, we are discussing relative risk.
Nicotinic stomatitis is a visible change to the hard palate. In response to chronic stress, the ortho-keratinized epithelium produces more keratin, leading to a more white-ish appearance. However, epithelial cells of the minor salivary glands do not respond to stress in this fashion, and remain pinkish (the genes for keratin production are possibly methylated and packed around histones in glandular epithelial cells). This same pattern can also be caused by ingesting hot liquids, but is acute and temporary in comparison to nicotinic stomatitis. Other than visual change, nicotinic stomatitis is usually asymptomatic and may be unknown to the patient.
Clinical changes to the gingiva
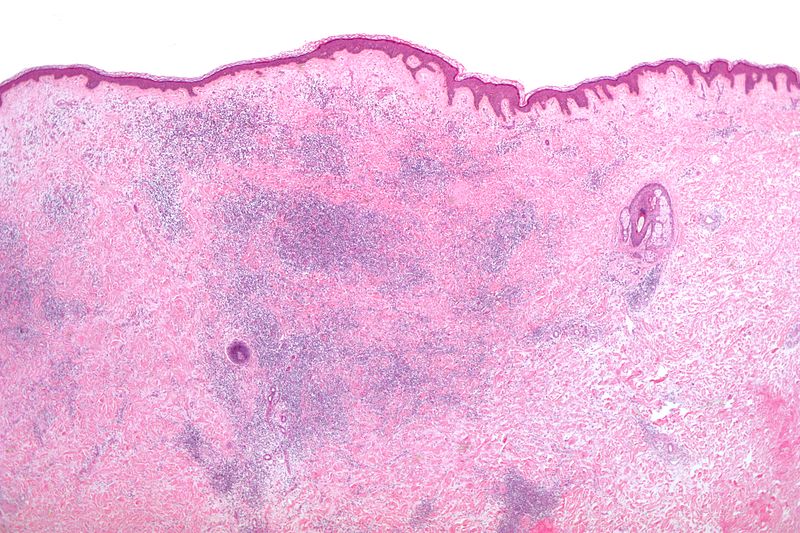
"Dermal perivascular lymphoeosinophilic infiltrate - very low mag" by Nephron is licensed under CC BY-SA 3.0Inflammation of any issue is referred to as tissue-name-itis, hence gingivitis is inflammation of the gingiva, while periodontitis is inflammation to the periodontium. The redness, swelling, heat and pain symptoms indicate the body has likely suffered trauma, and is undergoing a response to that trauma. Capillaries exude more liquid into an area as a part of the inflammatory process, known as edema. This makes inflamed regions of oral mucosa thicker and paler. Edema may give gingiva a puffy or rolled appearance.
Ideally, an inflammatory response limits the spread of the initial damage. After inflammation has prepped the injured area, a tissue can undergo regeneration. First, stem cells← migrate into the affected area, undergo mitosis←, and differentiate← into cells needed to repair the damage, such as keratinocytes or fibroblasts. Sometimes, these cells will produce an intermediate form of tissue first, such as granulation tissue, that will be remodeled afterwards.
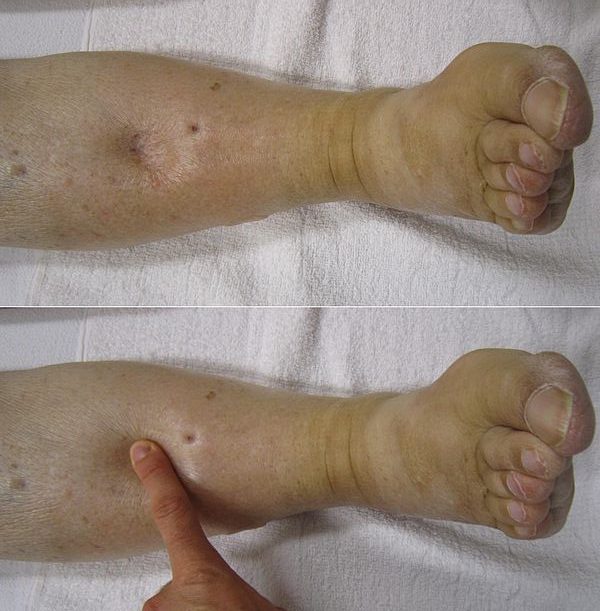
"Pitting edema" by James Heilman, MD is licensed under CC BY-SA 3.0Chronic inflammation, on the other hand, often leads to cell death and the loss or recession of a tissue. This is because stem cells← generally halt progression through the cell cycle until the inflammatory process removes the source of the stress. Without stem cells generating new cells, chronic stress allows everyday wear on a tissue to accumulate.
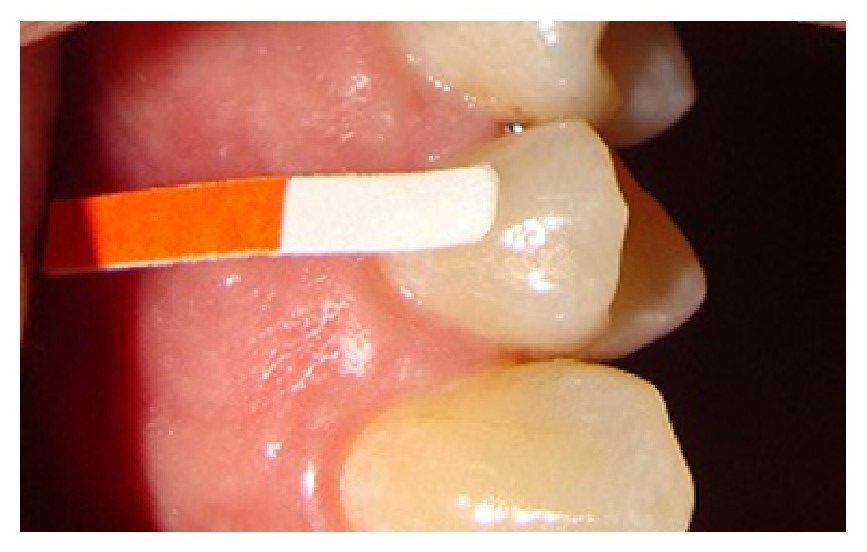
"Extracrevicular GCF collection" by Zeyad Nazar Majeed et al is licensed under CC BY 4.0During an inflammatory response, the gingival sulcus fills with gingival-crevicular fluid (GCF, or gingival-sulcular fluid). GCF contains breakdown products of human cells undergoing necrosis or apoptosis←, the breakdown products of bacteria being killed by white blood cells, bacterial toxins, and inflammatory molecules released by human cells. Taking a sample of gingival fluid as a diagnostic tool for measuring gingival health should be considered.

"Really bad gingivitis” by D. Rosenbach, is licensed CC BY 3.0Gingivitis
Gingivitis is any inflammation of the gums. It includes edema in the ECM of connective tissues in the lamina propria and sub-mucosa, as well as inside the epithelial cells of the oral mucosa. This causes the marginal, attached and interdental gingiva to become visibly swollen. When marginal gingiva is swollen, it may produce a crescent-shaped edema known as a McCall’s festoon. This inflammatory response generally causes no damage, but untreated gingivitis can progress to periodontitis, which in turn can lead to bone and tooth loss.
Research suggests gingivitis increases the risk of developing Alzheimer’s Disease and heart disease, making it all the more important to intervene as early as possible. Currently, 70% of Americans over the age of 65, 50% over the age of 30 and 80% of school-aged children suffer from periodontal disease.
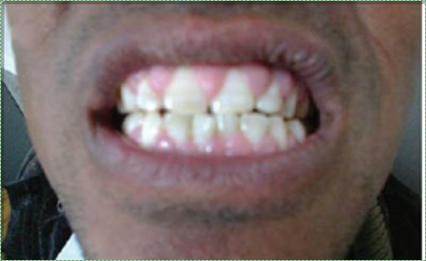
"Gingival enlargement due to S - amlodipine” by the NIH, is in the Public Domain CC0Gingival hyperplasia
Hyperplasia means the increased growth of a tissue, which could mean an increased number of cells, an increased amount of ECM produced by cells, or both. Gingival hyperplasia is an abnormal growth of gingival tissue. It may look similar to edema, but the underlying cause (and therefore treatment) is different. A tissue that has undergone hyperplasia will be dense, not squishy as a tissue with edema. Hyperplasia can be a side-effect of certain medications, such as phenytoin and cyclosporine. Other triggers exist, including pregnancy, hormonal disturbances, or it may even be a hereditary condition (Hereditary Gingival Fibromatosis). Like edema, gingival hyperplasia may be caused by poor oral hygiene. The first response of the immune system to oral microorganisms is inflammation (and therefore edema). Over time, the immune system may switch to releasing growth factors that trigger cell division of nearby stem cells←, and other signals (morphogens←) that trigger cell differentiation← and activity (such as the production of ECM proteins). This is a perfectly healthy response to stress in the palms of the hands or soles of the feet, generating a callus. It is also not bad way to shed parasites. Hoever, the body does not have separate mechanisms for reacting to physical stress and other types of stress, such as chemical stress caused by toxins produced by microorganisms. Increased growth of gingival tissue does not remove toxins or microorganisms.
Keep in mind some patients’ response to poor oral hygiene is gingival recession (covered next), not hyperplasia, for reasons described under edema. This seemingly irreconcilable difference results from the complexity of the immune system. Both gingival recession and hyperplasia are triggered by signaling molecules from white blood cells. While blood cells are, in turn, a large group of different cells that respond to an even larger variety of environmental stimuli (most diseases, physical trauma, allergens, tumors, parasites and more) by releasing a wide variety of chemical signals called cytokines. Cytokines produce a wide variety of different responses, including gingival hyperplasia in some patients and gingival recession in other patients. We encourage you to take a look at the list of different cytokines (Table 1) provided in the previous link before you come across the tables of different morphogen← signaling molecules we cover in chapters 6 through 11.
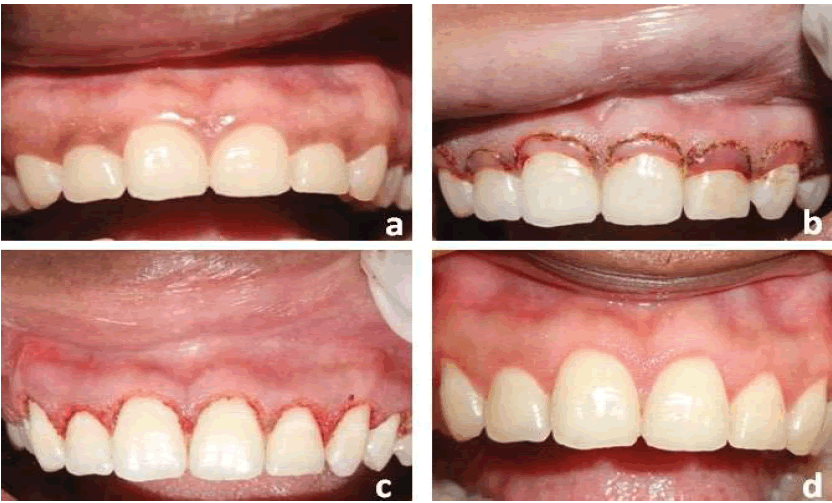
Esthetic crown lengthening” by Shanmukha Srinivas Manikanta Kumar Tirumalasetty et al is licensed under CC BY-NC 4.0Gingival hyperplasia should be addressed even when not caused by poor oral hygiene, as it may make maintaining oral hygiene difficult. In some cases, it may not be possible to remove the underlying cause (such as a hereditary condition, or a life-saving anti-seizure medication), in which case surgical removal of gingival tissue (gingivectomy) or gingival reshaping (gingivoplasty) may be warranted. Excess gingival tissue can be removed using a scalpel or a laser tool. The use of lasers can reduce bleeding and pain by cauterizing damaged blood vessels and ablating nerve endings.
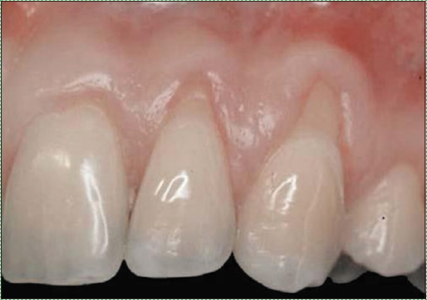
"Class II gingival recession on the left maxillary canine and lateral incisor” by Nitin Khuller, is licensed under CC BY 3.0Gingival recession
Chronic inflammation of the gingiva can lead to gingival recession (receding gums), exposing deeper tissues of the tooth, which in turn may make teeth more susceptible to tooth decay. The most common cause of gingival recession is periodontitis. Gingival recession can also be caused by abrasion (improper tooth brushing), abfraction (bruxism), improper tooth position, and sometimes aging (depending on the tissue biotype, host response and genetics).
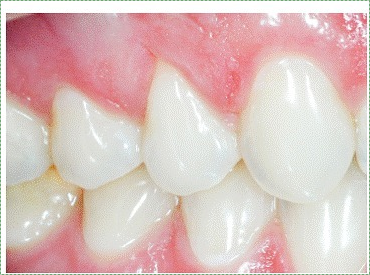
"V-shaped gingival recession” by Ana Suzy Jati et al, is licensed CC BY 3.0A Stillman cleft is a V-shaped region of gingival recession. It is often caused by occlusal trauma. Other etiologies include gingival inflammation and improper tooth brushing and flossing techniques. Under the microscope, the gingival tissue found bordering the Stillman cleft will appear fibrous and full of white blood cells, the typical response of a connective tissue to inflammation (Fig 3.27).
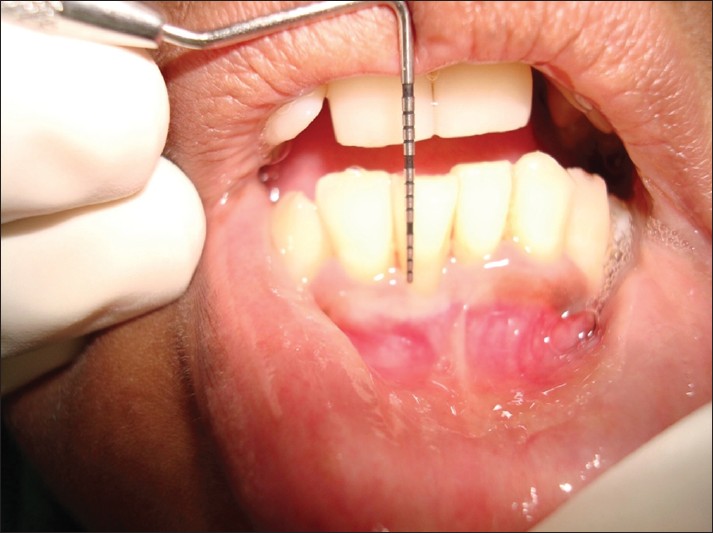
Tension test
During the intraoral exam, a tension tet may be performed by pulling the lip forward and moving it left and right. This helps to identify mucogingival defects. Mucogingival involvement are areas where there is gingival recession or loss of attachment of the gingiva. Loss of attachment causes the gingival margin to be freely moveable like the alveolar mucosa, not attached to underlying sub-mucosa and bone tissue. Healthy connections between rete pegs and dermal papillae prevent movement of the attached gingiva.
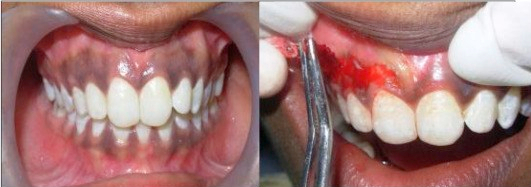
"Pre-operative view and Maxillary pigmentation removal using scalpel surgical technique" by Rehab A.Abdel Moneim et al is licensed under CC BY-NC-ND 4.0Pigmentation of the gingiva
Similar to increased levels of keratin in the attached gingiva, increased levels of melanin may obscure underlying healthy level of hemoglobin. The presence of melanin alone should not be mistaken for unhealthy gingiva. Unlike keratin, melanin is a pigment, meaning it absorbs only certain wavelengths of light (has a color). Surgeries and treatments to remove gingival pigment exist, often advertising what is found in textbooks: healthy gingiva should be pinkish or whitish in color (try a google search for gingival hyperpigmentation if you are skeptical). It is worth noting skin whitening products exist because of systemic racism in the United States and other countries has led many people of color to feel displeasure with darker skin pigmentation, illustrated by experiments such as the Doll test by Drs Kenneth and Mamie Clark. Furthermore, the concept that pink is healthy and brown is unhealthy is not shared across cultures. For instance, dental offices in Ethiopia and surrounding areas may offer ethnobotanical tattooing of the maxillary gingiva to add a blue-ish or grey-ish coloration to the gingiva, masking pinkish regions.
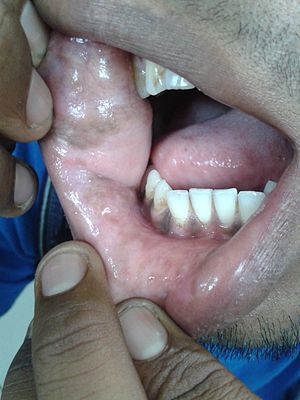
"Smoker's melanosis in oral mucosa with brown black pigmentation" by Skinstudy is licensed under CC BY SA 3.0A rapid, focal change in melanin production in the oral cavity may be a response to a medical condition. Most common among dark-skinned women between the ages of 30-50, increased melanin production in response to acute trauma or prolonged irritation (such as smoking) may arise, termed a melanocanthoma (further reading can be found here). Despite the name this type of skin lesion has, it is not a tumor, but a homeostatic change in cell activity. Tobacco smoke creates numerous free radicals that damage epithelial cells. Melanin can bind to free radicals and prevent them from reacting with DNA or lipids, thus increased melanin production helps protect the oral mucosa.
Tetracycline antibiotics bind to melanin, which triggers melanocytes to up-regulate melanin synthesis. Tetracycline drugs are very common, found in over-the-counter first-aid creams, added to animal feeds, and were even in beers brewed in Sudan 2,000 years ago.
There are other pigments that cause color changes to the gingiva, and they are classified as either endogenous (produced by human cells) or exogenous (environmental). Endogenous pigments commonly found in the gingiva, besides melanin, include hemoglobin. A reddish lesion caused by hemoglobin or its breakdown products is a sign of damage to capillaries (bruising). An example of an exogenous pigment is jimsonweed (Datura stramonium), which can be used in the ethnobotanical gingival tattoos mentioned previously.
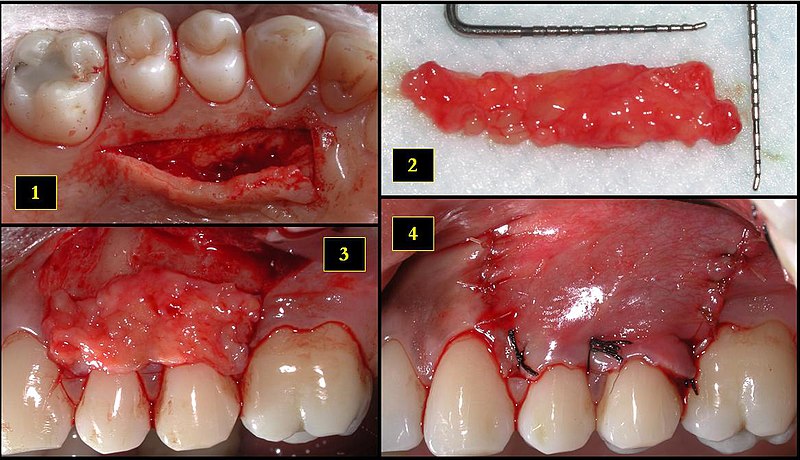
"Retrieval of the subepithelial connective tissue graft from the palate and placement at the recipient site" by DRosenbach is licensed under CC BY-SA 4.0Gingival grafting
A sub-epithelial connective tissue graft (SECT graft) may be performed to repair gingival recession. Unlike a skin graft, which grafts epithelial plus some or all of the connective tissue from a donor, a sub-epithelial graft transplants only connective tissue from the lamina propria and/or sub-mucosa. The goal of a sub-epithelial graft is not to replace damaged tissue, but to provide a scaffold← that promotes healing of the patient’s own tissues. The connective tissue contains collagen←, fibronectin← and hyaluronic acid to which epithelial stem cells← are attracted to and migrate over (thanks to integrins← and other trans-membrane protein receptors). These stem cells undergo mitosis←, producing more epithelial cells which differentiate← into keratinocytes and regenerate the oral epithelium. Similarly, the patient’s mesenchymal stem cells migrate through the scaffold and replace the transplanted collagen, regenerating the lamina propria and sub-mucosa. Connective tissue grafts are relatively common in dentistry and maxillofacial surgery, but these technologies are starting to generate changes in tissue grafting below the neck, too.
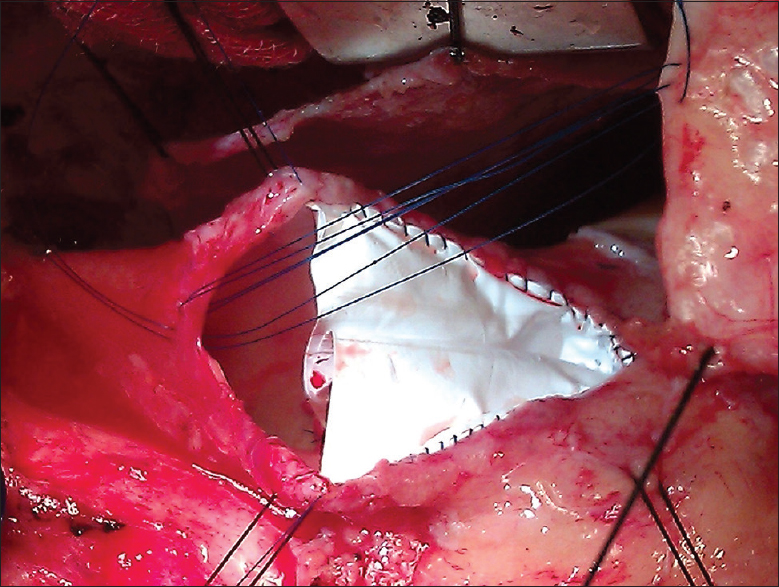
Intraoperative view showing hand sewn polytetrafluoroethylene bicuspid pulmonary valve in situ with transannular pericardial patch being sutured" by Prashant Ramdas Wankhade et al is licensed under CC BY-NC-SA 4.0Sub-epithelial grafts splice connective tissue from nearby regions of healthy gingiva (such as from neighboring gingiva or hard palate). This leaves behind small wounds, which heal quickly. Nevertheless, damaging healthy tissue is not optimal. Another option is to use tissue from a human cadaver. Because the connective tissue used in such a procedure is mostly collagen← fibers, other options include the use of synthetic collagen-like polymers (Fig 3.38) or a pericardial patch procedure. Unlike human tissue, there is a large supply of cow and pig connective tissue. First, the connective tissue that surrounds a cow or pig heart (the pericardium) is harvested and stripped of any pig or cow cells. The acellular tissue that remains should not trigger tissue rejection. Collagen shares a very high degree of homology across vertebrate species, meaning our collagen is nearly identical to cow and pig collagen. Just like in SECT, collagen acts as a scaffold←. Ultimately, the patient’s own epithelial stem cells← migrate over the scaffold to regenerate the oral epithelium. In addition, the patients mesenchymal stem cells from nearby healthy lamina propria and sub-mucosa migrate through the scaffold, and replace cow or pig collagen with human connective tissue. This procedure is similar to types of bone tissue grafts and is not limited in use to the gingiva—a video can be viewed here of a heart valve replacement using bovine pericardium. We hope you appreciate the amount of histology and cell biology required to understand how pieces of cow hearts can be used to repair damaged gingiva.
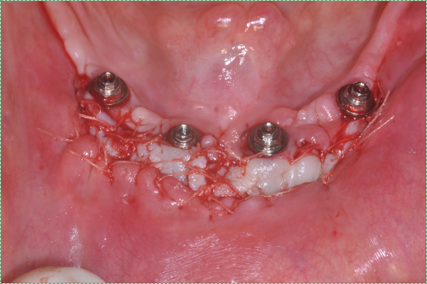
"Free gingival graft placed around the implants” by Danny Omar Mendoza Marin, is licensed CC BY 3.0A gingival graft (such as a free gigival graft or other related procedures) may be placed around dental implants←, or used to repair gingival recession. Similar to the sub-epithelial graft, a gingival graft harvests healthy tissue from the donor, only in this case epithelial cells from the oral mucosa are transplanted along with connective tissue. When placed around dental implants←, attached gingival tissue can adhere to the implant, similar to junctional epithelium which adheres to a tooth. The attachment may contain hemi-desmosomes, however it does not form a pocket with a thin epithelium– this difference in morphology will become more apparent after learning about the development of junctional epithelium during tooth eruption←. Coating a dental implant with hyaluronic acid helps grafted tissue adhere to the implant. Without adhesion to the implant, oral microorganisms bypass the oral mucosa and enter the sub-mucosa.
| For further reading on dental biomaterials |
|---|
FDA information on GINTUIT |
Mucograft – acquired by Geistlich Biomaterials |
Gengigel hyaluronic acid gel |
Clinical applications of dento-gingival junction histology
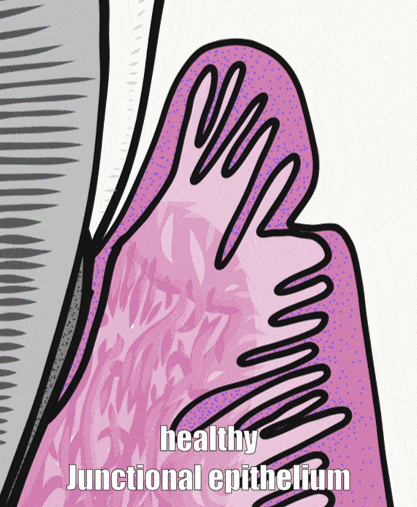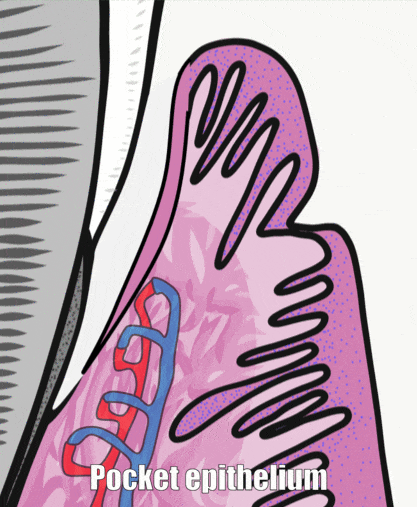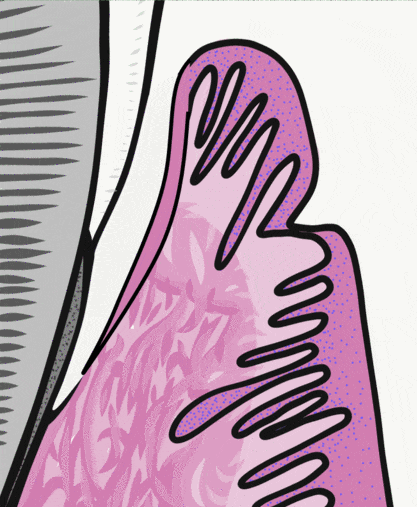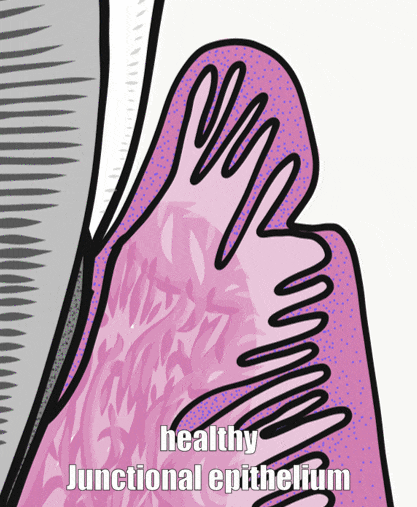
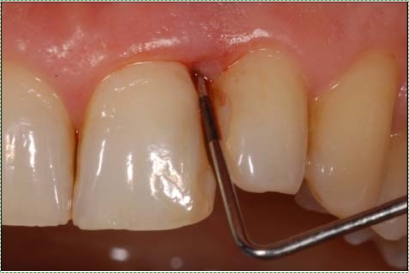
Periodontal pockets
The depth of a periodontal pocket can be measured using a calibrated probe. In a healthy state, the distance from the marginal gingiva to the epithelial attachment should be between 1 to 3 mm. Pockets within this range typically have an intact epithelial attachment, which prevents oral bacteria from entering the sub-mucosa and causing gingivitis or periodontitis. Poor oral hygiene, however, can lead to increased levels of oral bacteria within the periodontal pockets. Because junctional epithelium is more permeable than other regions of oral mucosa, white blood cells come into contact with this bacterial population and trigger inflammation. With chronic inflammation comes a loss of junctional epithelium, which can reduce the thickness of the junctional epithelium further, potentially causing the loss of the epithelial attachment. At this point, the pocket is said to be lined with pocket epithelium. Since it is no longer attached to the tooth, the probe can likely be inserted over 3 mm. The thinness of the pocket epithelium brings the probe closer to blood vessels in the lamina propria, making it more likely to damage these vessels, causing bleeding on probing (BoP).
Bleeding after probing" by Luigi Checchi et al, is licensed CC BY NC 3.0The major risk with bleeding on probing involves the oral microbiome, which is a large collection of microorganisms. These microorganisms come into contact with the bloodstream with disruption to the epithelial barrier. Even so-called “good bacteria” trigger inflammation when they move inside the body. Until damage to the barrier is repaired, inflammation continues. Chronic inflammation within the pockets leads to damage to nearby tissue, such as alveolar bone. This leads to further damage to the oral cavity which we discuss later. A second risk with migration of the epithelial attachment is that acid-producing bacteria now come into contact with softer cementum, leading to root caries.
It is possible for a periodontal pocket deeper than 3 mm to have junctional epithelium with an intact epithelial attachment. These exhibit minimal bleeding on probing, and can be considered pseudopockets rather than a clinical manifestation of periodontitis. Pseudopockets, which may also be called false pockets or gingival pockets, are caused by gingival hyperplasia or edema. Enlargement of the gingival margin, whether by hyperplasia or edema, deepens the measurement of a pocket, but does not share the same harmful loss of the epithelial attachment. The underlying cause of the pseudopocket may need to be addressed, especially if there is bleeding on probing.
Clinical applications of specialized mucosa histology
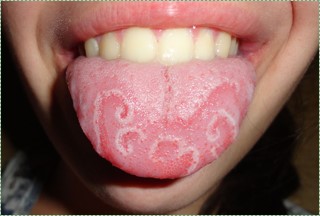
"geographic tongue" by Jbarta, is licensed CC BY SA 3.0Geographic tongue
Geographic tongue is a condition where filiform papillae on the dorsal surface of the tongue become non-uniformly hyper-keratinized, giving some filiform papillae a more white-ish appearance. Other papillae may be lost due to prolonged inflammation, leaving more reddish spots on the tongue. The pattern of keratinized versus partially-keratinized papillae changes over weeks. The result is almost always a cosmetic concern, although some patients may describe periods of heightened sensitivity to hot, acidic and/or spicy foods. There are currently no treatments for geographic tongue. The underlying causes of geographic tongue are unknown.
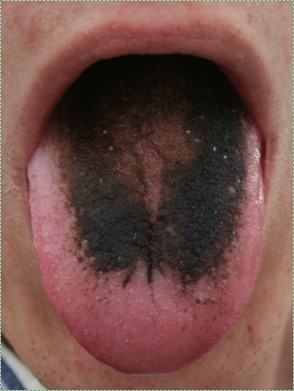
black hairy tongue" by Com4, is in the Public Domain CC 0Black hairy tongue
Black hairy tongue occurs when filiform papillae exfoliate epithelial cells more slowly, thus papillae become enlarged from the buildup of cells. This allows the papillae to pick up more stains from tobacco smoke, foods, or oral bacteria, creating thicker, darker bumps on the tongue. It is thought that this condition might be triggered by overgrowth of certain oral fungi, possibly following the loss of competition with the use of certain antibiotics. The reason the filiform papillae appear hair-like is that both filiform papillae and hairs are composed predominantly of dead keratinized epithelial cells. Patients are encouraged to brush their tongue when brushing their teeth.
< Chapter 2 * navigation * Chapter 4 >
Chapter review questions
The mucous membrane lining the inside of the mouth. It is a stratified squamous epithelium, named the oral epithelium, and an underlying areolar connective tissue named the lamina propria.
The developmental history of a differentiated cell traced back to the embryonic cell from which it arises.
he most exterior of the three primary germ layers formed in the gastrula, it gives rise to the epithelial tissue covering the body and the CNS.
The middle of the three embryonic layers that develop during gastrulaton, mesoderm becomes most of the body's muscle and connective tissues.
Epithelia that have more than one layer of cells, with the cells at the apical surface having a flat shape.
A loose connective tissue composed of fibroblasts and other cell type, plus ground substance and all three fiber types.
A connective tissue that has fibers that are not arranged in parallel bundles as in dense regular connective tissue. Dense irregular connective tissue consists of mostly collagen fibers. It has less ground substance than loose connective tissue.
Th connective tissue layer of the skin, found just deep to the epidermis, composed of areolar CT and dense irregular CT.
Fingerlike projections of the apical side of the dermis, increasing the surface area between the epidermis and dermis, strengthening their connection and increasing exchange of oxygen, nutrients, and waste.
The top (or apex) side of a cell or tissue, usually facing the lumen.
Fingerlike projections of the epidermis, the downward-pointing counterparts to the dermal papillae.
The superficial epithelial layer of the skin, composed of a stratified squamous epithelium.
Having the protein keratin, which lends resistance to abrasion and water loss.
One of a family of tough fibrous structural proteins, the key material making up hair, nails, calluses, and the outer layer of skin.
The main structural protein in the ECM of connective tissues
the gelatinous material within a living cell, excluding organelles.
A terminally differentiated epithelial cell capable of synthesizing large amounts of the protein keratin within its cytoplasm.
Undifferentiated or partially differentiated cells that can differentiate into various cell types, and proliferate to produce more of the same stem cell.
The side of a cell or tissue oriented away from the lumen or external surface.
Without blood vessels
Trans-membrane structure specialized for cell-to-cell adhesion.
The stratified squamous epithelium of the oral mucosa.
A group of red, dark brown or black pigments found in the hair, skin, and other places, capable of absorbing UV-B radiation.
Terminally differentiated cells derived from neural crest cells that produce one of the three forms of melanin.
When one cell begins to look different from another. This process involves limiting cell fate by altering gene transcription to become more specialized.
A temporary group of cells that arise from the embryonic ectoderm, and in turn give rise to a diverse cell lineage—including melanocytes, cranio-facial cartilage and bone, teeth and periodontal tissue, smooth muscle, peripheral and enteric neurons and glia.
The embryonic precursor to the central nervous system.
Terminally differentiated and highly specialized cells of neural tissue, capable of firing electrical signals over long distances and releasing chemical signals across discrete locations called synapses.
The numerous and diverse group of cells in neural tissue that provide support for, guide the activity of, and organize the connections between neurons.
Cell Adhesion Molecules: proteins located on the cell surface involved in binding with other cells or with the extracellular matrix.
A structure inside (or outside) a cell, consisting of liquid enclosed by a lipid bilayer.
A sequence of nucleotides in DNA that encodes the synthesis of a gene product, either RNA or protein.
Heritable DNA modifications that do not change the DNA sequence but do affect gene activity.
Copying DNA into a functional product, such as a RNA and/or protein. Controlled by the activity of transcription factors binding to gene promoter regions to recruit RNA polymerase.
deoxyribonucleic acid is the hereditary material in humans and almost all other organisms.
The embryonic process in which one group of cells directs the development of another group of cells.
Signaling molecules secreted directly into the blood which travel throughout the body.
A medical condition that is present at or before birth, which can be acquired during development or from the genetic make up of the parents.
A congenital malformation involving incomplete closure of the neural tube.
A molecule made by Red Blood Cells that can bind and release Oxygen molecules. It switches from a red to a maroon color as it does so, which in turn contributes to skin color.
An orange-to-yellow pigment made by plants that accumulates within the dermis of the skin. It is the precursor for Vitmain-A.
A substance whose non-uniform distribution governs the pattern of tissue development and pattern formation.
Methyl groups added to DNA which change the activity of a gene without changing the sequence, typically repressing gene transcription.
Highly basic proteins found in nuclei that compact DNA into a denser form, unavailable for gene transcription.
The lip, as opposed to adjacent skin or oral mucosa, named for its more reddish appearance than keratinized skin.
An infolding of the epidermis that extends deep into the dermis, responsible for producing a hair.
Microscopic exocrine glands in the skin that open into hair follicles to secrete an oily or waxy matter, called sebum, which lubricates the hair and skin.
The process by which animals and plants grow and change.
Ca5(PO4)3(OH), the inorganic ECM material composed of calcium, phosphate and hydroxide ions that makes up the bulk of bone tissue.
Inward folding of an epithelium caused by interstitial growth.
The folding process in vertebrate embryos, which includes the transformation of ectoderm into the neural tube.
A polarity axis that organizes cells in the plane (side-to-side) of the tissue.
A general term for peeling or shedding, in humans it refers to the loss of dead epithelial cells or structures (hair, teeth).
rough Endoplasmic Reticulum, an organelle where membrane and secreted proteins are translated by bound ribosomes on the surface of the rER.
A membrane-bound organelle that modifies proteins from the rER and packages them up into vesicles, to be sent to lysosomes, the plasma membrane, or secreted from the cell.
Whitish-yellow bumps found on the lips or oral mucosa, caused by the unusual presence of sebaceous glands (or possibly minor salivary glands) trapped below the epidermis.
The three layers of cells that arise from gastrulation: endoderm, mesoderm and ectoderm.
A similarity due to shared lineage between a pair of structures or genes in different taxa.
The areolar connective tissue layer of the oral mucosa (or hollow organ), homolgous to the papillary layer of the dermis.
The dense irregular connective tissue layer found below the oral mucosa (or mucosa of a hollow organ), homologous to the reticular layer of the dermis.
A stratified squamous epithelium that does not contain the protein keratin, making it softer and moister than the skin.
A non-keratinized stratified squamous epithelium found in the buccal mucosa, labial mucosa, alveolar mucosa, ventral surface of the tongue, floor of the mouth, and soft palate.
Thin protein fibers in the ECM, capable of stretching and returning to their original length, made of the protein elastin.
Areas of oral mucosa that have become partially keratinized due to the friction and abrasion of the masticatory process, including the gingivae and hard palate.
Region of oral mucosa firmly bound to the tooth and alveolar process.
An epithelium that is partially keratinized and contains visible nucleuses.
An epithelium that is partially keratinized that does not contain visible nucleuses.
Border between the mucosa of the cheeks and floor of the mouth-- which are freely moveable and fragile-- and the the mucosa around the teeth and on the palate-- which are firm and keratinized.
The epithelium found on the dorsal surface of the tongue, containing lingual papillae and taste buds.
The top surface of the tongue, containing lingual papillae and taste buds.
Bumps on the tongue, giving it its rough texture
The oral mucosa of the lips (both inner and outer surface)
The lining mucosa of the cheeks and inner side of the lips.
A white line. In the oral cavity, refers to a line in buccal mucosa along the occlusal plane.
In the phrase nature vs nurture, nurture means environmental factors that occur after fertilization, not DNA inherited from our parents.
In the phrase nature vs nurture, nature refers to genetics (heritable traits).
The bottom surface of the tongue, which contains no lingual papillae.
Portion of the oral cavity underneath the tongue.
The posterior muscular portion of the roof of the mouth.
The lining mucosa covering the alveolar ridges, attached to the buccal, lingual and palatal mucosa in one direction, and gingival mucosa in the other direction.
The part of the jaws that holds the teeth.
Collagen fibers in the gingiva that, in general, attach the tooth to the gingival tissue (rather than the tooth to the alveolar bone like the PDL fibers).
Portion of the attached gingiva between teeth, coronal to the free gingival margin on the buccal and lingual surfaces of the teeth
A 1.5 mm strip of gingival tissue which surrounds the neck of the tooth.
The horizontal bony plate that makes the anterior portion of the palate of the mouth, composed of parts of the palatine and maxilla bones.
A hard connective tissue which is mostly ECM, including collagen fibers and a calcium-phosphate crystal.
Epithelium lining the gingival sulcus.
Space between the gingiva and tooth.
Epithelium on the inner wall of the gingiva that attaches to connective tissue of the lamina propria on the basolateral side and to the surface of the tooth on the apical side.
Half of a desmosome, specialized for cell-to-ECM adhesion.
located at the bottom of the sulcus, consisting of approximately 1 mm of junctional epithelium and 1 mm of gingival fiber attachment.
Containing blood vessels
A series of externally visible anterior tissue bands lying under the early brain that give rise to the structures of the head and neck.
A soft tissue whose cells contains a large amount of actin and myosin proteins, allowing it to change in size (contraction and elongation).
A type of connective tissue composed primarily of adipocytes, which store high-energy triglyceride and other lipid molecules.
V-shaped groove separating the anterior two thirds of the tongue from the posterior third and containing the circumvallate papillae.
A signaling molecule capable of stimulating cell proliferation, wound healing, and cellular differentiation.
An abnormally high level of keratin production in an epithelium, causing a thickening and whitening of the outer layer of the skin or oral mucosa.
Whitish patches or lesion of the oral mucosa caused by hyper-keratinization.
The process of cell division, where one cell divides into two identical clones (daughter cells).
Agents or factors which cause malformation of an embryo.
Periodontal Ligament, a group of specialized connective tissue fibers that attach a tooth to alveolar bone.
Hyper-keratinization of the hard palate mucosa-- but not minor samilvary gnds-- caused by cigarette smoke or other stresses,.
Small salivary glands located throughout the oral cavity that generally get no name.
Inflammation of gingival tissue
Inflammation that damages soft tissue and bone that anchors the teeh, including the gingiva plus PDL, cementum or alveolar bone tissue.
Specialized tissues that surround and support the teeth, including gingival tissue, PDL, cementum and alveolar bone.
Swelling caused by excess fluid trapped within tissues.
After partial loss of as tissue, based on the remaining part, the tissue grows the same structure and function as the lost part.
The basic connective tissue cell type capable of secreting ECM components, including fibers and ground substance proteins.
Reorganization or renovation of existing tissues, either physiological or pathological. The process can either change the characteristics of a tissue such as in blood vessel remodeling, or result in the dynamic equilibrium of a tissue such as in bone remodeling.
Fluid exudate from gingival mucosa that collects within the gingival sulcus. Presence of bacterial enzymes, bacterial degradation products, connective tissue degradation products, inflammatory molecules, or extracellular matrix proteins can be detected in higher levels in gingival crevicular fluid during the active phase of periodontitis.
Programmed cell death
Extra-Cellular Matrix: Materials in the body, but outside of cells. A network of macromolecules, such as fibers, enzymes, and glycoproteins, that provide structural and biochemical support to surrounding cells.
Semilunar-shaped enlargements of the marginal gingiva primarily on the labial surfaces of the anterior and premolar teeth.
Growth of a tissue by an increased number of cells, an increased amount of ECM produced by cells, or both.
Overgrowth of gum tissue around the teeth, often associated with poor oral hygiene.
The exposure in the roots of the teeth caused by a loss of gum tissue and/or retraction of the gingival margin from the crown of the teeth.
a broad and loose category of small proteins (~5–20 kDa) important in immune syhstem responses, including chemokines, interferons, interleukins, lymphokines, and tumour necrosis factors, but generally not hormones or growth factors (despite overlap in the terminology). Cytokines are produced by a broad range of cells, including immune cells like macrophages, B lymphocytes, T lymphocytes and mast cells, as well as endothelial cells and fibroblasts.
A V-shaped region of gingival recession
the cause, set of causes, or manner of causation of a disease or condition
having an internal cause or origin; made by human cells.
Having an external cause or origin; not made by human cells
The most common method used to treat root exposure, involving the transplantation of healthy connective tissue from a donor site to the damaged area, which acts a scaffold aiding in tissue regeneration.
Extracellular matrix material involved in the repair of injured and missing tissues, allows stem cells to migrate into the injured area. It is usually replaced during regeneration.
A large glycoprotein found in the ECM which binds to integrin proteins on the cell surface, involved in cell adhesion, growth, migration, and differentiation.
A very large glycosaminoglycan distributed widely throughout connective tissue ECM, epithelial, and neural tissues.
A trans-membrane protein which allows cells to bind to the ECM protein fibronectin, it is involved in cell adhesion, growth, migration, and differentiation.
Proteins embedded within the plasma membrane, with parts both the inside and outside of the cell.
A cell-surface, trans-membrane or cytoplasmic protein that binds to (receives) a signaling molecule and transmit the signal further.
Multipotent cells of a connective tissue that can differentiate into a variety of cell types, including osteoblasts, fibroblasts, chondrocytes, myoblasts, blood cells and adipocytes.
A medical procedure hat grafts connective tissue from cow or pig pericardium to human tissues to boost healing and regeneration.
A medical procedure in which ground up bone tissue or synthetic bone tissue is placed in an injured site to act as a scaffold, promoting the formation of new bone tissue by the patient's own cells.
The use of connective and epithelial tissue from the roof of the mouth or neighboring gingiva, attached to the gum area being treated.
An artificial tooth root that is surgically placed into bone tissue.
The shape (or form) of.
A process in tooth development in which the teeth enter the mouth and become visible.
A pathologically deepened gingival sulcus around a tooth at the gingival margin.
The thin, overly-permeable epithelium that replaces junctional epithelium in a periodontal pocket.
Bleeding that is induced by gentle manipulation of the tissue at the depth of the gingival sulcus.
A lesion located on the root surface of a tooth, usually close to or below the gingival margin.
A pocket depth over 3mm caused by enlargement of the gingival margin, but does not have harmful loss of the epithelial attachment.
A condition where filiform papillae on the dorsal surface of the tongue become non-uniformly hyper-keratinized, creating dynamic regions of more white and more pink coloration.
Fingerlike bumps on the dorsal surface of the tongue that contain epithelium for friction, but no taste buds.
A temporary, harmless condition that gives the tongue a dark, furry appearance, resulting from a buildup of dead skin cells on the papillae on the dorsal surface of the tongue.

