14.3 Sea-Floor Sediments
Except within a few kilometers of a ridge crest, where the volcanic rock is still relatively young, most parts of the sea floor are covered in sediments. This material comes from several different sources and is highly variable in composition, depending on proximity to a continent, water depth, ocean currents, biological activity, and climate. Sea-floor sediments (and sedimentary rocks) can range in thickness from a few millimeters to several tens of kilometers. Near the surface, the sea-floor sediments remain unconsolidated, but at depths of hundreds to thousands of meters (depending on the type of sediment and other factors) the sediment becomes lithified.
The various sources of sea-floor sediment can be summarized as follows:
- Terrigenous sediment is derived from continental sources transported by rivers, wind, ocean currents, and glaciers. It is dominated by quartz, feldspar, clay minerals, iron oxides, and terrestrial organic matter.
- Pelagic carbonate sediment is derived from organisms (e.g., foraminifera) living in the ocean water (at various depths, but mostly near surface) that make their shells (a.k.a. tests) out of carbonate minerals such as calcite.
- Pelagic silica sediment is derived from marine organisms (e.g., diatoms and radiolaria) that make their tests out of silica (microcrystalline quartz).
- Volcanic ash and other volcanic materials are derived from both terrestrial and submarine eruptions.
- Iron and manganese nodules form as direct precipitates from ocean-bottom water.
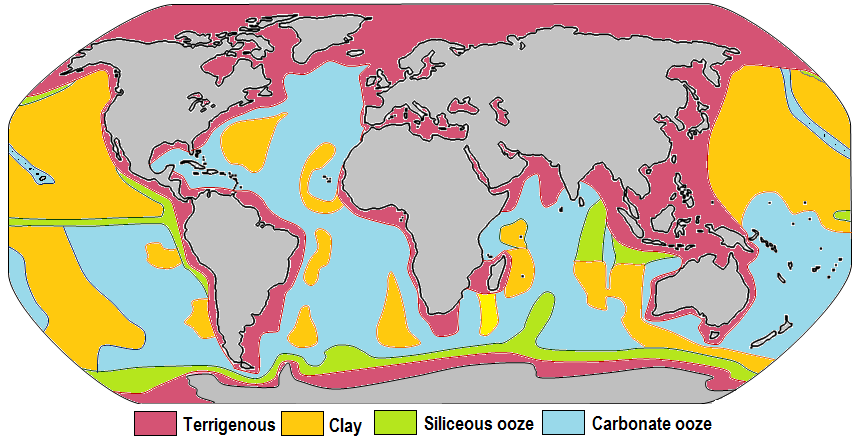
The distributions of some of these materials around the seas are shown in Figure 14.11. Terrigenous sediments predominate near the continents and within inland seas and large lakes. These sediments tend to be relatively coarse, typically containing sand and silt, but in some cases even pebbles and cobbles. Clay settles slowly in nearshore environments, but much of the clay is dispersed far from its source areas by ocean currents. Clay minerals are predominant over wide areas in the deepest parts of the ocean, and most of this clay is terrestrial in origin. Siliceous oozes (derived from radiolaria and diatoms) are common in the south polar region, along the equator in the Pacific, south of the Aleutian Islands, and within large parts of the Indian Ocean. Carbonate oozes are widely distributed in all of the oceans within equatorial and mid-latitude regions. In fact, clay settles everywhere in the oceans, but in areas where silica- and carbonate-producing organisms are prolific, they produce enough silica or carbonate sediment to dominate over clay.
Carbonate sediments are derived from a wide range of near-surface pelagic organisms that make their shells out of carbonate (Figure 14.12). These tiny shells, and the even tinier fragments that form when they break into pieces, settle slowly through the water column, but they don’t necessarily make it to the bottom. While calcite is insoluble in surface water, its solubility increases with depth (and pressure) and at around 4,000 meters, the carbonate fragments dissolve. This depth, which varies with latitude and water temperature, is known as the carbonate compensation depth, or C.C.D. As a result, carbonate oozes are absent from the deepest parts of the ocean (deeper than 4,000 meters), but they are common in shallower areas such as the mid-Atlantic ridge, the East Pacific Rise (west of South America), along the trend of the Hawaiian/Emperor Seamounts (in the northern Pacific), and on the tops of many isolated seamounts.

Exercise 14.3 What type of sediment?
The diagram shows the sea floor in an area where there is abundant pelagic carbonate sediment. There is a continent within 100 kilometers of this area, to the right. What type of sediment (coarse terrigenous, clay, siliceous ooze, or carbonate ooze) would you expect at find at locations a, b, c, and d?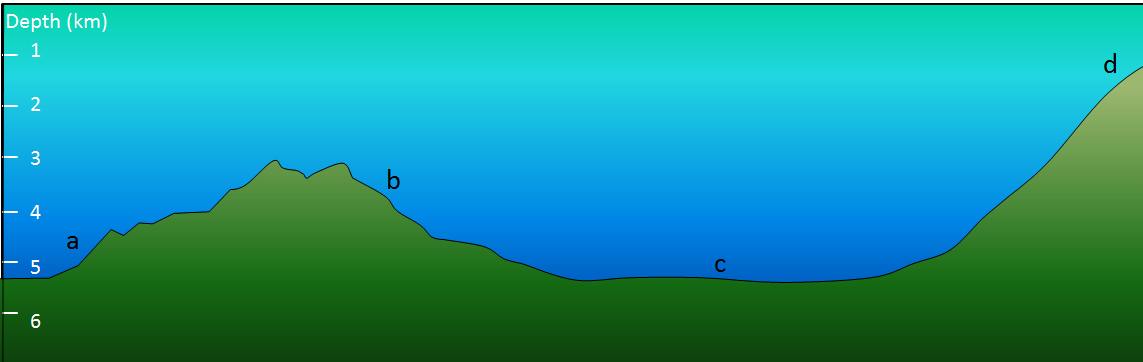
Figure 14.13 Figure shows typical locations for the deposition of marine sediments. A. is farthest from the continent. D is closest to the continent.
- A depth of 4.5 kilometers.
- A depth of 3.5 kilometers.
- A depth of 5 kilometers.
- A depth of 1 kilometer, close to the edge of a continent.
All terrestrial erosion products include a small proportion of organic matter derived mostly from terrestrial plants. Tiny fragments of this material plus other organic matter from marine plants and animals accumulate in terrigenous sediments, especially within a few hundred kilometers of shore. As the sediments pile up, the deeper parts start to warm up (from geothermal heat), and bacteria get to work breaking down the contained organic matter. Because this is happening in the absence of oxygen (a.k.a. anaerobic conditions), the by-product of this metabolism is the gas methane (CH4). Methane released by the bacteria slowly bubbles upward through the sediment toward the sea floor.
At water depths of 500 meters to 1,000 meters, and at the low temperatures typical of the sea floor (close to 4°C), water and methane combine to create a substance known as methane hydrate. Within a few meters to hundreds of meters of the sea floor, the temperature is low enough for methane hydrate to be stable and hydrates accumulate within the sediment (Figure 14.14). Methane hydrate is flammable because when it is heated, the methane is released as a gas (Figure 14.14). The methane within sea-floor sediments represents an enormous reservoir of fossil fuel energy. Although energy corporations and governments are anxious to develop ways to produce and sell this methane, anyone that understands the climate-change implications of its extraction and use can see that this would be folly. As we’ll see in the discussion of climate change in Chapter 16, sea-floor methane hydrates have had significant impacts on the climate in the distant past.

Video: Earth Rocks – Ocean Sediments
Media Attributions
- Figure 14.11, 14.12, 14.13: © Steven Earle. CC BY.
- Figure 14.14 (Left): “Gashydrat im Sediment” © Wusel007. CC BY-SA.
- Figure 14.15 (Right): “Burning Gas Hydrates” by J. Pinkston and L. Stern (USGS). Public domain.
Licenses and Attributions
“Physical Geology – 2nd Edition” by Steven Earle is licensed under CC BY 4.0 Adaptation: Renumbering, Remixing
a single-celled protist with a shell that is typically made of CaCO3
the shell-like hard parts (either silica or carbonate) of small organisms such as radiolarian and foraminifera
photosynthetic algae that make their tests (shells) from silica
microscopic (0.1 to 0.2 millimetres) marine protozoa that produce silica shells
the depth in the ocean (typically around 4000 metres) below which carbonate minerals are soluble
processes that take place without oxygen
a combination of water ice and methane in which the methane is trapped inside “cages” in the ice

