5.3 Amine Protonation
In amines, nitrogen is bonded to three other atoms (alkyl groups or hydrogens) and has a lone pair of nonbonding electrons. This lone pair produces a region of electron density on the nitrogen atom, which coupled with the electronegativity of nitrogen produces a partial-negative charge at that location. In samples containing amines there are intermolecular attractive forces between the amine nitrogen and other partially-positive or positive particles.
Bronsted-Lowry theory describes acids as Hydrogen ion (proton) donors. The hydrogen ion, H+, is one type of positively-charged particle that can interact with an amine. By associating with the N and utilizing the lone pair for bonding, the amine can convert from an uncharged species to one that carried a positive charge. This takes the H+ from solution and incorporates it into a cation structure, called an ammonium ion:
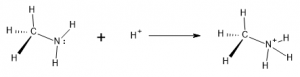
Even at a neutral pH of 7, enough acid is available in solution for the ammonium ion to predominate. At lower pH, more acidic solutions it becomes almost the exclusive form.
Since the free, unprotonated amine is able to pick up or ‘accept’ a hydrogen ion in this reaction, the free amine (reactant above) is classified as a base. Various amines will exhibit this behavior to a greater or lesser degree, depending on the specific structures surrounding the amine functional group. For instance, molecules of aromatic amines with nitrogen attached are less commonly in the base form and are thus considered weaker bases.
Ammonium ions exhibit properties that clearly differ from the free, uncharged amine. These ions are more water soluble and can form ionic bonds with various anions to produce ionic substances that can be solid at room temperature. Most small unprotonated amines, by contrast, are liquids.
Amines are abundant in nature and appear very commonly in biologically-active molecules, whether those come from natural sources (like plants) or man-made ones (like pharmaceuticals). Liquid medicines are difficult to handle; producers and consumers alike appreciate the long shelf-stability and easy storage of drugs in the form of pills. Whereas an unprotonated amine may be an oily liquid under normal conditions, the corresponding ammonium ion is often part of an ionic solid which can be formed into a tablet. So pharmaceutical companies can use pH adjustment to help them formulate drugs into a better form. Also, the transport of drug molecules in the body will be influenced by water-solubility, so this solubility switch can be important for that reason, too.
Exercise 5.3.1
Exercise 5.3.2
Provide a description in your own words that explains why a substance that is soluble in water at pH=6 might be insoluble at pH=9.

