7.2 Aldehydes and Ketones
Aldehydes
An aldehyde can be recognized by noting the presence of a carbonyl group at the end of a carbon chain. Consider the line-bond structure of the three carbon aldehyde ‘propanal’ here:
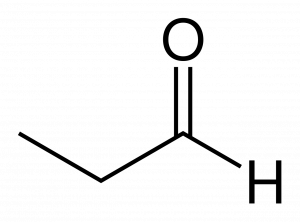
Hydrogen is shown explicitly on the aldehyde group for clarity, as it usually is. Note that because the aldehyde functional group includes both the double bond to oxygen and the hydrogen bonded to the carbonyl carbon, this group must exist at the end of the carbon chain: it requires 3 of 4 shared pairs of electrons from a carbon.
Aldehyde names are quite similar to the alcohols they resemble, with key modifications. For instance, the molecule above with a hydroxy group rather than an aldehyde group would be named 1-propanol. The suffix ‘-ol’ given to an alcohol is replaced with the suffix ‘-al’ to identify this is an aldehyde. The number becomes unnecessary because the aldehyde group can only be at position 1 on the parent chain. So it is not used.
In a branched aldehyde the parent chain begins at the carbonyl carbon. Naming structures with multiple functional groups can get tricky, but freely-available IUPAC rules provide guidance.
The presence of the aldehyde group can influence the properties of substances, due to the polarity that exists when this group is present. Aldehydes in mixtures with other substances can engage in hydrogen bonding as the oxygen can act as a hydrogen-bond acceptor. In pure samples, however, the aldehyde can not hydrogen-bond because there is no highly-polarized bond to a hydrogen. The hydrogen attached to the carbonyl is not very polarized, because the carbonyl carbon itself is electron deficient as a result of its bond to oxygen.
Exercise 7.2.1
Exercise 7.2.2
Ketones
When a carbonyl exists within a chain of carbons the resulting structure is called a ketone. For instance here are three images representing the substance known by the common name ‘acetone.’ First is a line-bond structure, then a ball-and-stick style structure, and finally a picture of the substance as you would find it in the laboratory:
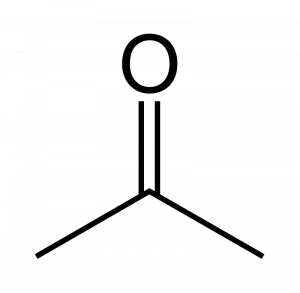

The ketone functional group includes a carbonyl carbon connected to two additional carbons. It is a carbonyl that is positioned within a chain of carbons.
Ketones are named using the suffix ‘-one.’ Acetone has an IUPAC name of propanone. In larger ketones a location for the ketone group also needs to be included in the name, since multiple locations are possible:
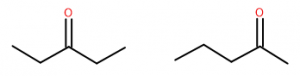
What names would be given to each of the two compounds shown above? These two substances have very similar but distinct characteristics. Both have a molecular dipole, but other than the carbonyl itself both are entirely hydrocarbon, so they are just somewhat polar. Their physical properties including melting and boiling points are affected by this, as well as their ability to dissolve in various solvents.
So while similar in many ways, they are slightly different and are, in fact, different substances. They are structural isomers, and their names are 2-pentanone and 3-pentanone.
IUPAC rules guide us to number the parent chain so that the keto group the lowest possible number. Once the parent chain has its carbons numbered, any substituent groups are numbered accordingly.
Exercise 7.2.3

