7.4 Esters and Amides
Esters
Esters are one type of carbonyl compound described as carboxylic acid derivatives. In this group the ‘-OH’ group of a carboxylic acid has been replaced by a group containing a carbon-containing, ‘-OR’ group. As an example:
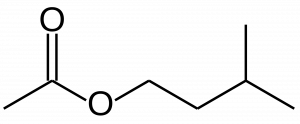
This ester is 3-methylbutyl ethanoate. It is also known by its common name, isoamyl acetate. This substance is one of a few fragrance/flavor compounds that distinguish the flavor of banana, though it is also found in some other fruits. Artificial banana flavor, such as that used to make popular candy, is largely composed of this substance.
Note the carbonyl here, and the second oxygen atom connected directly to the carbonyl carbon. These structures look very similar to the carboxylic acid structures, but the properties of esters do not include acidity. Esters are thus unable to ionize, making them less water-soluble than carboxylic acids. And while un-ionized carboxylic acids are able to engage in intermolecular hydrogen bonding, esters are not. So esters are also less polar, and have lower melting and boiling points than their counterpart carboxylic acids.
Many flavor and fragrance compounds from flowers and fruits are esters. Plant material is  immensely complex, but individual esters separated out from plant extracts can be so characteristic of plant odors that people to identify their sources specifically. Plant fragrances in nature are often produced as animal attractants, and as animals we have evolved sensory organs that can identify the presence of these substances and enjoy many of them.
immensely complex, but individual esters separated out from plant extracts can be so characteristic of plant odors that people to identify their sources specifically. Plant fragrances in nature are often produced as animal attractants, and as animals we have evolved sensory organs that can identify the presence of these substances and enjoy many of them.
The intermediate polarity of esters, coupled with their somewhat small (for an organic chemical) size means that many have low enough boiling points to have some of these molecules escape into the gas phase near their source. From where they are formed–from a flower or a piece of fruit–those molecules can drift into our noses.
Esters tend to be somewhat reactive, decomposing into other substances when heated or exposed to dramatic variations in pH. Cooking can alter the flavor of foods by contributing to the destruction of these compounds.

The names of esters are based on two separate sections of these molecules, and unlike most IUPAC names are given as two separate words. The first is based on the organic structure beyond the carbonyl, ‘-methyl’ or ‘-ethyl’ etc.. In the case of our banana oil compound, this is the ‘3-methylbutyl’ portion of the name. The second word in the name is derived from the carboxylic acid that would exist were we to remove the carbons beyond the carbonyl and replace them with a hydrogen. In the example here we have ‘-ethanoate,’ which comes from ‘ethanoic acid,’ the two-carbon carboxylic acid.
Exercise 7.4.1
Amides
Amides are also carboxylic acid derivatives. These compounds have a carbonyl directly connected to a nitrogen. That nitrogen can be bonded to hydrogens or to additional carbons. The relationship between this group and the amines is suggested in the name “amide.”
Naming can get quite complicated for amides, but the simplest amides are named based on the carboxylic acid they most closely resemble, with ‘-oic acid’ in the carboxylic acid name converted to ‘-amide.’
For instance, ethanamide looks like this:
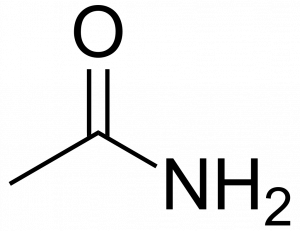
Unfortunately for students, the IUPAC system is often cast aside for familiar names when chemists discuss amides. This substance is commonly called acetamide, a non-systematic, common name.
Amides have electronegative nitrogen atoms, with their lone pair electrons in a position close to the carbonyl group. This arrangement produces molecules with some polarity, but the degree of polarity in an amide is also influenced by what connects to the nitrogen itself. In short, polarity can vary a lot within this family of compounds. Ethanamide, shown above, can engage in hydrogen-bonding interactions, and has properties that reflect that. But the more highly substituted amides with additional carbons attached at the nitrogen are not able to do so, and have properties that correspond:
Resistance to rotation around the amide bond
Amides tend to share some electron density with the carbonyl unit itself, causing the C-N bond to exhibit some double-bond character. In particular, for amides located in the middle portion of larger molecules, these groups lock into a conformation illustrated below, resisting rotation around the carbon to nitrogen bond. This is similar to the lack of rotation we learned about in the carbon to carbon double bond (such as in cis-trans geometric isomers).
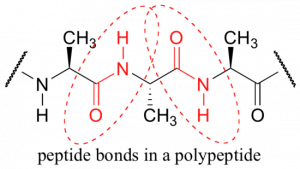
In 3-dimensional molecules and models, the amide adopts a planar (flat) configuration, and the flexiblity of the whole molecule is vastly reduced. This locked structure is occurs in multiple locations in proteins, where amino acids which bond to one another do so via amide linkages also known as peptide bonds. Larger structural features of proteins, such as the secondary structure units ‘alpha helix’ and ‘beta sheet’ frequently discussed in biochemistry, form in part because of this particular feature of the amide bonds within those proteins.
Exercise 7.4.2

