2.3 Isomers
Constitutional isomers
Imagine you were asked to draw a structure for a compound with the molecular formula C4H10. This would not be difficult – you could simply draw:
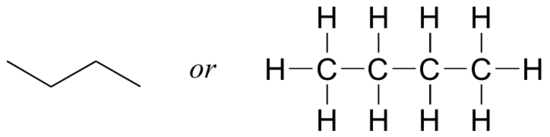
But when you compared your answer with that of a classmate, they may have drawn this structure:
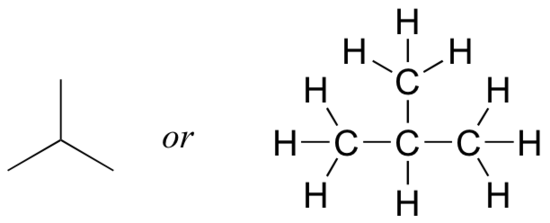
Who is correct? The answer, of course, is that both of you are. A molecular formula only tells you how many atoms of each element are present in the compound, not what the actual atom-to-atom connectivity is. There are often many different possible structures for one molecular formula.
Compounds with this relationship: the same molecular formula but different connectivity, are called constitutional isomers (sometimes the term ‘structural isomer’ is also used). The Greek term ‘iso’ means ‘same.’
The word isomer is a relational word, that describes the relationship between two things. It is similar in that sense to the word ‘cousin’ or ‘uncle.’ Being a cousin is not an identity essential to you, but describes your relationship to someone else. A substance can be an isomer to something else, but being an isomer is not an essential, internal characteristic.
Fructose and glucose, two kinds of sugar molecules, are related as constitutional isomers of one another. Can you figure out the molecular formula for each?
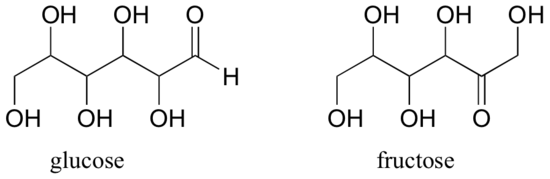
We will learn about other kinds of isomers including those that have the same molecular formula and the same connectivity in later chapters.
Exercise 2.3.1
How would you explain the difference between the structures of glucose and fructose? Would you make the explanation to a chemist (or fellow chemistry student) differently than you would make it to someone who doesn’t have any background knowledge of the subject?
Exercise 2.3.2
Exercise 2.3.3
Draw a constitutional isomer of ethanol, CH3CH2OH. If you are unable to draw it, explain it with words.
Exercise 2.3.4
Exercise 2.3.5
Draw as many constitutional isomers with the given molecular formula as you can, for each formula given. If you are unable to draw them, describe them with words.
a) C5H12
b) C4H10O
c) C3H9N

