2.1 Drawing and Interpreting Organic Formulas
Organic molecules can be large and can contain multiple atoms of carbon, hydrogen and other elements. Their architecture can be complicated, with chains of various lengths and ring structures.
Consider the structures shown here:
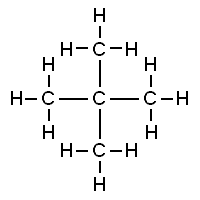
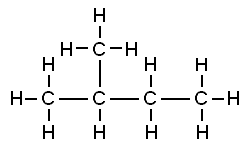
Both of these structures are representations of organic molecules. Each contains 5 carbon atoms and 12 hydrogen atoms. But they look different, and it turns out they are: the properties of these substances are similar but not exactly the same.
The substance to the left has a boiling point of 9.5 ºC, making it a gas at room temperature. The substance to the right has a boiling point of 28 ºC, so it is a liquid at room temperature.
As we begin to consider and study organic molecules we very quickly are faced with this fact: molecular structures (showing the number and types of atoms in a molecule, such as H2O) are inadequate to describe these substances.
In organic chemistry we make use of different types of formulas for this reason. There are several types, including
- Structural formulas: which show every atom with its elemental symbol and every bond drawn as a line. These look a lot like the Lewis Structures you probably learned to draw in Introductory Chemistry 1, but without the nonbonding electron pairs.
- Condensed structural formulas: similar to the structural formulas but without bonds shown to hydrogen atoms, so a carbon with 3 hydrogens attached becomes a CH3. There are various levels of condensed, and there are some rules about how formulas are properly condensed. You will read these more often than write them since all these rules are challenging to learn.
- Line-bond, also called Skeletal structures: These are the hardest to learn but the quickest to draw of those described here. They are used extensively in chemical communication. In line-bond structures all bonds between carbons, and between carbons and other atoms except hydrogen are shown. Hydrogens are not included unless they are attached to something other than carbon. And the carbon atoms are shown without the elemental symbol. Instead the viewer recognizes carbon as present anywhere there is a vertex (pointed place) in the drawing, and at the end of a line.
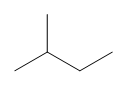
The structures above are shown as structural formulas, but could also be represented as condensed or line-bond structures. Can you tell which of the two structures is shown in the examples here?
CH3CH(CH3)CH2CH3
Common bonding patterns in organic structures
Drawing structural formulas is a good starting point for a novice organic chemist, and works when dealing with small, simple structures. But when you start dealing with larger structures it becomes increasingly difficult and time-consuming. Imagine trying to draw all atoms and bond every time you wanted to discuss the structure below, which is one small piece of DNA:
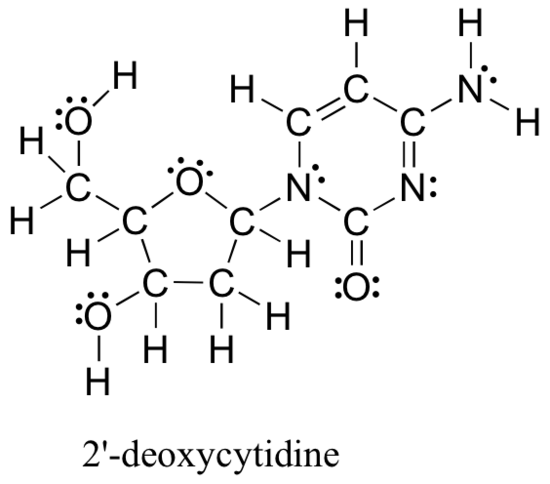
Large molecules such as this are commonly considered in organic chemistry and biochemistry. In these situations Line-bond structures really help. To get good at drawing them accurately, you will first want to get familiar with some common bonding arrangements involving elements found frequently in organic molecules.
Let’s start with carbon. Carbon is said to be tetravalent, meaning that it tends to form four bonds. If you look at a variety of structures including carbon, you can see that nearly always each carbon atom has four bonding pairs of electrons, each represented as a line, surrounding it.
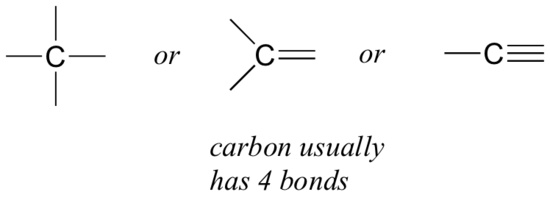
This is a pattern that holds throughout most of the organic molecules we will see.
If a carbon has other electron arrangements in its valence shell (in other words, if it does not fulfill the octet rule), it will have a formal charge or exist as a radical:
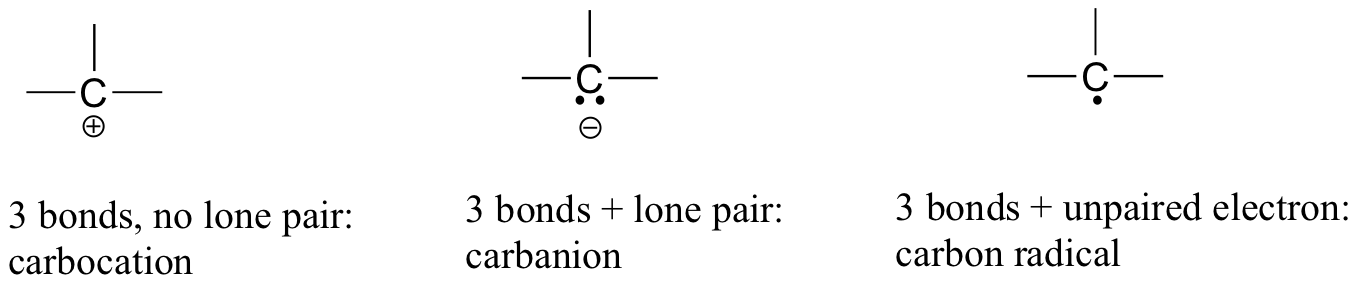
If you are able to quickly recognize these patterns (and the patterns described below for other atoms) it will help you tremendously as you learn more about organic chemistry.
The pattern for hydrogens is easy: hydrogen atoms have only one bond, and no formal charge. As a rule, all hydrogen atoms in organic molecules have one bond, and no formal charge.
For oxygen, you will see an the atom bonding in three ways, all of which fulfill the octet rule.
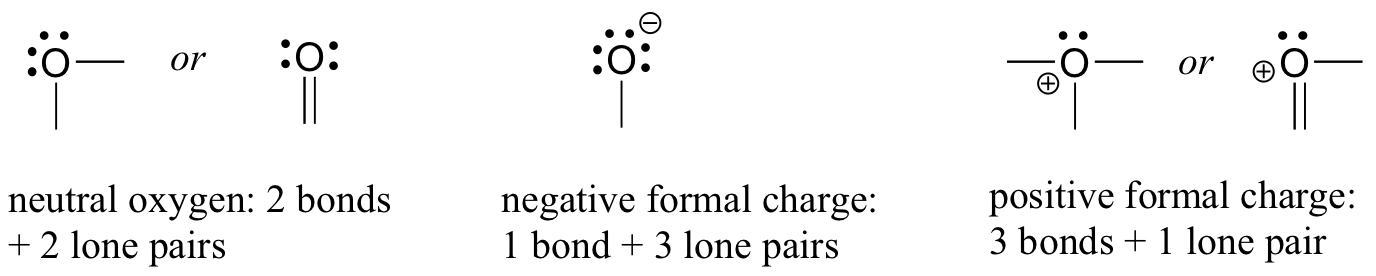
In most cases an oxygen atom has two bonds and two lone pairs, as in does in water. In this arrangement it will have a formal charge of zero. If it has one bond and three lone pairs, as in hydroxide ion, it will have a formal charge of-1. If it has three bonds and one lone pairit will have a formal charge of +1.
There are, again, some additional possibilities. However these three examples will account for virtually everything we see.
Nitrogen has two major bonding patterns, both of which fulfill the octet rule:
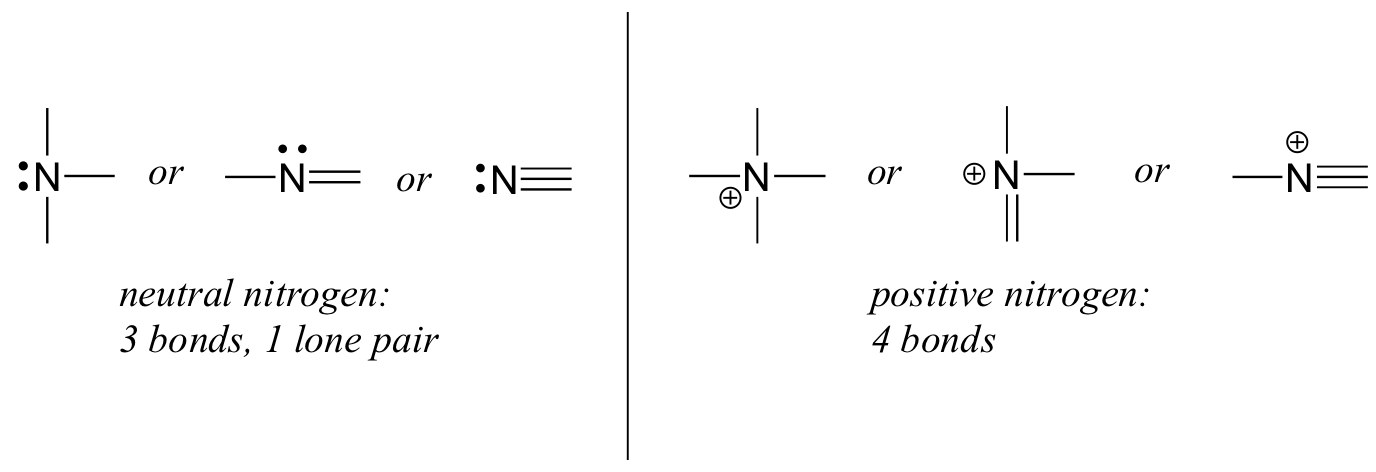
If a nitrogen has three bonds and a lone pair, it has a formal charge of zero. If it has four bonds (and no lone pair), it has a formal charge of +1. In a fairly uncommon bonding pattern, negatively charged nitrogen has two bonds and two lone pairs.
Two third row elements are commonly found in important organic molecules: sulfur and phosphorus. Although both of these elements have other bonding patterns that are relevant in laboratory chemistry, in a biological context sulfur almost always follows the same bonding/formal charge pattern as oxygen, while phosphorus is present in the form of phosphate ion (PO43-), where it has five bonds (almost always to oxygen), no lone pairs, and a formal charge of zero. Remember that atoms of elements in the third row and below in the periodic table have ‘expanded valence shells’ with d orbitals available for bonding, and the the octet rule does not always apply.
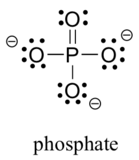
Finally, the halogens (fluorine, chlorine, bromine, and iodine) are very important in laboratory and medicinal organic chemistry, but are less common in naturally occurring organic molecules. Halogens in organic compounds usually are seen with one bond, three lone pairs, and a formal charge of zero. Sometimes, especially in the case of bromine, we will encounter reactive species in which the halogen has two bonds (usually in a three-membered ring), two lone pairs, and a formal charge of +1.
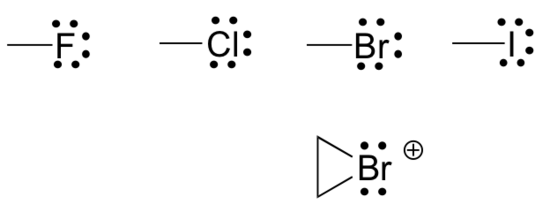
These rules, if learned and internalized so that you don’t even need to think about them, will allow you to draw large organic structures, complete with formal charges, quite quickly.
Organic structure types often do not include lone pairs, since you can assume that the proper number of electrons are present around each atom to match the indicated formal charge (or lack thereof). Occasionally, though, lone pairs are drawn if doing so helps to make an explanation more clear.
Using the ‘line bond structure’ convention
If you look ahead in this and other books at the way organic compounds are drawn, you will see that the figures are somewhat different from the Lewis structures you are used to seeing in your general chemistry book. In some sources, you will see condensed structures for smaller molecules instead of full structural formulas that include every bond:
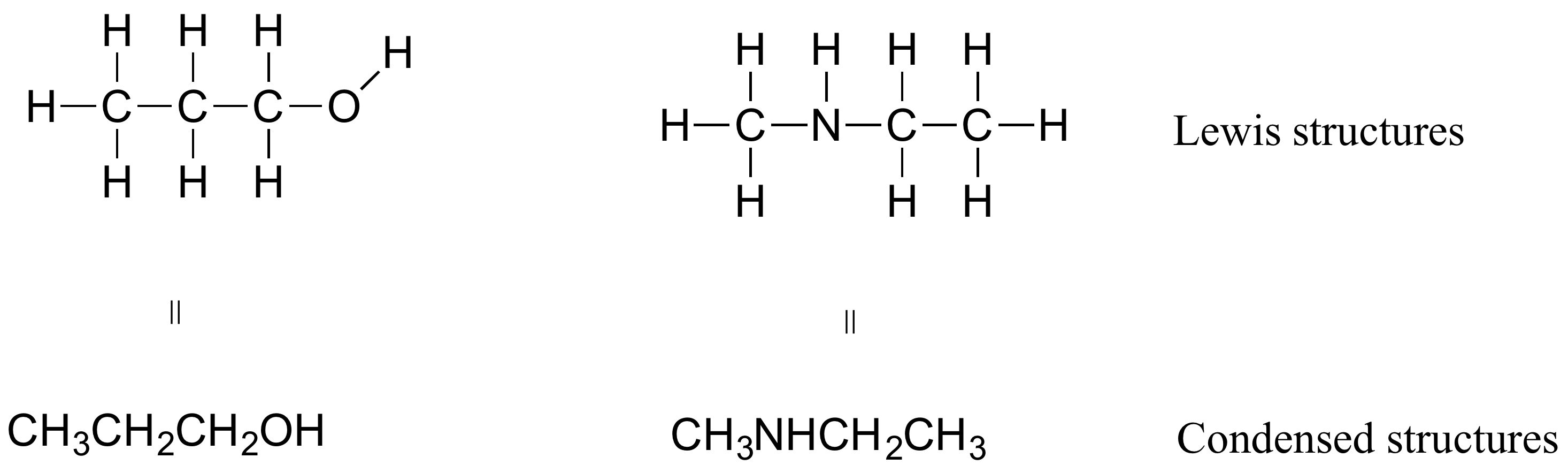
More commonly, organic and biological chemists use an abbreviated drawing convention called line-bond structures, also sometimes called skeletal structures. The convention is makes it easier to draw molecules, but the convention does need to be learned.
Here is basically how it works: Carbon atoms are not depicted with their elemental symbol, but rather by a vertex (corner) or a free end of a bond. Open-chain molecules are usually drawn out in a ‘zig-zig’ shape. Hydrogens attached to carbons are generally not shown: rather, like lone pairs, it is assumed the person viewing the structure knows where they are. Hydrogens bonded to nitrogen, oxygen, sulfur, or anything other than carbon are shown, but are usually drawn without showing the bond. The following examples illustrate the convention.
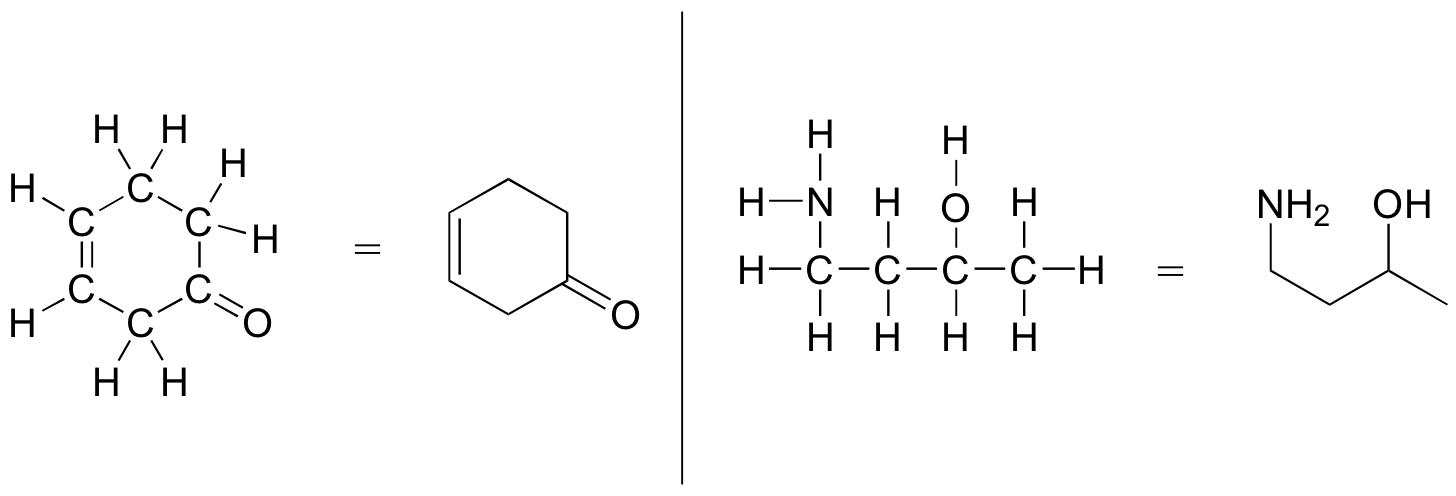
The line-bond structures makes it much easier to see the basic structure of the molecule and the locations where there is something other than C-C and C-H single bonds. It can also be drawn quickly.
Sometimes, one or more carbon atoms in a line structure will be depicted with a capital C, if doing so makes an explanation easier to follow. If you label a carbon with a C, you also must draw in the hydrogens for that carbon.
Exercise 2.1.1
A good way to test your understanding of the line structure convention is to see if you can accurately determine the number of hydrogen atoms in a molecule from its line structure. Do this for the structures here.
Exercise 2.1.2
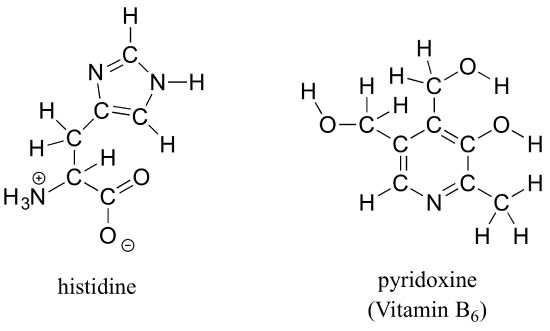
Draw line structures for histidine (an amino acid) and pyridoxine (Vitamin B6).
Exercise 2.1.3
Exercise 2.1.4
How many hydrogens are bonded to carbon at each hotspot (identified with a purple plus sign) on the structure shown below?

