9.1 Names and Structures for Halogenated Compounds
Elements in Group 17 are named the Halogens. These elements are well known for their ability to gain electrons and form anions in ionic compounds. But halogens also readily engage in covalent bonding. They form diatomic molecules as elements, such as chlorine gas (Cl2) and solid iodine (I2). They also commonly engage in covalent bonding with carbon. When they do, the resulting substances are classified as halogenated organic substances, also known as alkyl halides.
Halogenated organic substances are not common in biological system, though they do exist. Iodine, for example, is an important part of the thyroid hormone molecules including thyroxine. Our dietary need for small amounts of iodine are due to the need our body has to make these hormones. The iodine comes from either iodated salt, or from exposure to iodine near salt water.
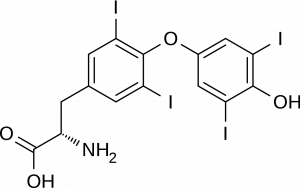
Halogenated hydrocarbons are useful in many industrial and consumer applications, so they are frequently synthesized. The halogenated alkene with the common name

vinyl chloride (chloroethene) is the monomer that is used to produce polyvinyl chloride, or PVC plastic. PVC is abundant in the built environment, where it is used commonly for pipes such as sewers, for structural elements such as window frames in homes, and in carpets and flooring materials. PVC is also the base material for vinyl siding.
Pharmaceuticals also frequently are halogenated organic compounds. Examples include the anti-anxiety drug Diazepam, which contains chlorine, and the antidepressant Fluoxetine, which contains fluorine. Pesticides and herbicides are also frequently organic molecules that contain halogen atoms.
Naming halogenated alkanes
Consider this structure:
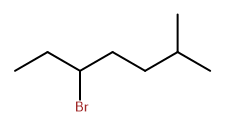
This halogenated alkane has a parent chain that is seven carbons long, so it will be named as a derivative of heptane. The halogen, bromine, might be assigned a location of 3, or a location of 5 if numbering from the opposite end of the chain. There is also a methyl group as a substituent on the chain. In this case IUPAC guides us to assign locations to give bromine the lowest possible number. So bromine is at position 3, the methyl group is at position 6, and the name is
3-bromo-6-methylheptane
Note that carbon 3 is surrounded by four different groups. This means that the carbon at that position can come in two alternative forms (R or S configured). Two stereoisomers of the substance, which are enantiomers, are possible. The structure below shows the S isomer:
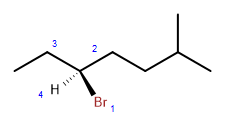
The numbers (in blue) indicate the priority of each of the four groups surrounding the chiral carbon. Using this system one can determine this is the specific stereoisomer designated as S. The mirror image of this compound, R-3-bromo-6-methylheptane, will have very similar properties to this substance but will be noticeably different in the way it behaves in chiral systems: it will rotate plane-polarized light in the opposite direction from the S isomer, and it would have different effects in biological systems.
The name of this isomer is thus complete when the R or S designation is included: this is (S)-3-bromo-6-methylheptane.
Can you draw the enantiomer of this substance?

