Appendix
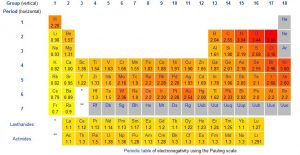
Electronegativities of the Elements, Pauling Scale
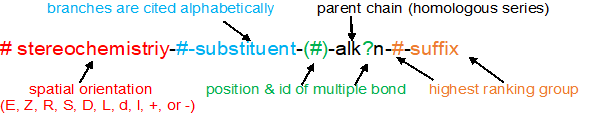
Overview of the IUPAC System for Naming Organic Compounds
Rules (current as of December 2020) for organic nomenclature from the International Union of Pure and Applied Chemistry (IUPAC) can be found at
https://www.acdlabs.com/iupac/nomenclature/
Summary Rules for Naming Organic Compounds
Taken from ChemLibre SCC Chem 420 text on Organic Chemistry
The International Union of Pure and Applied Chemistry (IUPAC) has established the rules of nomenclature of all chemical compounds. IUPAC nomenclature can also be called “systematic” nomenclature because there is an overall system and structure to the names. This section provides an overview of the general naming strategy and structure for organic compounds.
Naming organic compounds according to the IUPAC system requires up to four pieces of information
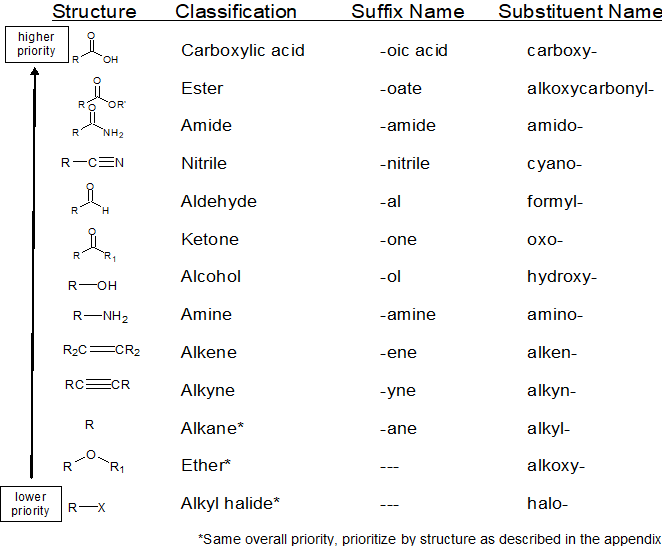
1. recognize & prioritize the functional group(s) present
2. identify & number the longest continuous carbon chain to give the highest ranking group the lowest possible number
3. cite the substituents (branches) alphabetically using the numbering determined above
4. recognize & classify any stereochemistry (E/Z, R/S, cis/trans)
With these four pieces of information, the IUPAC name is written using the format below. This same format applies to ALL the organic compounds.
Recognize and prioritize the Functional Group(s) Present
Identify & Number the Longest Continuous Carbon Chain with the Highest Priority Group
The longest continuous carbon chain (parent) is named using the Homologous Series, as well as any carbon branches. The suffixes and location within the name distinguish between the parent and the branches. Some of the homologous series names for saturated, straight-chain hydrocarbons are shown here, along with the substituent forms used when naming branches.
| Parent chain length | Alkane base name | Substituent form |
| 1 Carbon | Methane | Methyl |
| 2 carbons | Ethane | Ethyl |
| 3 carbons | Propane | Propyl |
| 4 carbons | Butane | Butyl |
| 5 carbons | Pentane | Pentyl |
| 6 carbons | Hexane | Hexyl |
| 7 carbons | Heptane | Heptyl |
| 8 carbons | Octane | Octyl |
| 9 carbons | Nonane | Nonyl |
| 10 carbons | Decane | Decyl |
| 11 carbons | Undecane | Undecyl |
| 12 carbons | Dodecane | Dodecyl |
When Alkenes and Alkynes have Lower Priority
The hydrocarbon suffixes differ in the letter preceeding the “n”. When alkenes and alkynes occur in compounds with higher priority functional groups, then the distinction between hydrocarbons is communicated with a single letter: “e”, or “y” for alkenes and alkynes, respectively, An example is shown below to illustrate the application of this rule.

Cite the substituents (branches) alphabetically using the numbering determined above
Substituent forms above are used when naming simple branches off the parent chain. Additional branching does occur, naming guidelines for these situations are not introduced here.
Recognize and Classify Stereochemistry
Distinguishing spatial orientations of atoms (stereochemistry) are communicated at the beginning of the name using the appropriate symbols, such as E/Z, R/S, cis/trans, etc..
Atoms or groups are called cis or trans to one another when they project respectively on the same or on opposite sides of a reference plane identifiable as common among stereoisomers. The compounds in which such relations occur are termed cis/trans-isomers. For doubly bonded atoms, the reference plane contains these atoms and is perpendicular to the plane containing the doubly bonded atoms and the atoms directly attached to them.
In names of compounds, steric relationships around one or more double bonds can be designated by the stereodescriptors Z and/or E , assigned as follows. The sequence-rule-preferred atom or group attached to one of a doubly bonded pair of atoms is compared with the sequence-rule-preferred atom or group attached to the other of that doubly bonded pair of atoms; if the selected atoms are on the same side of the reference plane the italic capital letter Z is used as the stereodescriptor; if the selected atoms are on opposite sides, the italic capital letter E is used as the stereodescriptor. These stereodescriptors, placed in parentheses followed by a hyphen, normally precede the whole name; if the molecule contains several double bonds, then each stereodescriptor is immediately preceded by the lower or less primed locant of the relevant double bond.
Chiral centers are labelled as R or S preceded when necessary by appropriate locants.

