8.2 Combustion
Our society depends on combustion reactions for providing some of our most basic needs: heat, light, and transportation. Additionally combustion reactions have strong cultural power for many people, who have grown up with the idea of a comforting hearth, backyard barbecues, campfires, and warm meals and drinks. Combustion reactions are perhaps among the most familiar of all chemical reactions, and yet we may go through life without giving much thought to their chemical nature.

Through a chemical lens, combustion is the reaction of a fuel with oxygen, to produce heat and light as well as chemical products. While some combustion reactants are not carbon-based, the most familiar of these reactions involve organic fuels such as wood, coal, gasoline, and other carbon-based substances. The oxygen required for reaction is most commonly drawn from the air. When carbon compounds combust in this way the main products of the reaction are carbon dioxide and water. Natural materials are complex mixtures, so other products can also form, and even the combustion of the organic fuel itself can include some side reactions producing other products. These biproducts may not be abundant but can be very important if they are released into the environment. They can have negative impacts on human health and the environment. So can carbon dioxide, of course, as it is perhaps the most important of the greenhouse gases that human activity adds to the atmosphere.
Organic combustion fuels
Just about any molecule with some hydrocarbon can combust. Combustion involves the oxidation of the carbon in an organic molecule. The fuels that are burned on a large scale to provide energy for heat or electricity include the fossil fuels: natural gas, petroleum products, and coal. Natural gas is primarily methane, and combusts according to this reaction:
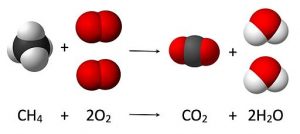
Other gaseous alkanes commonly used for fuel can be refined out of natural gas also, including ethane, propane, and butane.
Petroleum, also known as crude oil, is a liquid natural product with a composition that varies depending on the source. Petroleum is thus refined, leading to the production of many commercial products. Included in these are gasoline, kerosene, and fuel oil. While the specific equations describing the combustion of these substances varies based on the makeup of the reactant, the general pattern remains: fuel molecules react with molecular oxygen to produce carbon dioxide and water.
Coal, a solid fossil fuel, is also a mixture but combusts well due to the presence of hydrocarbons in the substance. Again, the substance we call coal is a mixture that varies depending on its source, but the combustion generally involves large hydrocarbon molecules, and the oxidation of those carbons through reaction with oxygen gas.
Among non-hydrocarbon fuels, ethanol has seen a lot of use. The development of ethanol as fuel in the United States occurred as new markets were sought for an abundance of starchy crop products, such as corn, during the past 50 years. Alcohols burn similarly to hydrocarbons, although the carbon within them is somewhat more oxidized to begin with. A consequence of this is that the energy yield per gram from combustion of alcohols tends to be somewhat lower than for hydrocarbons:
C2H5OH + 3 O2 ![]() 2 CO2 + 3 H2O
2 CO2 + 3 H2O
Ethanol has been touted as a greener fuel than the non-renewable fossil fuels. However the production of ethanol from commodity crops like corn is itself fossil fuel dependent, and analyses have shown that the production of ethanol does not actually reduce overall fossil fuel use as much as it may first appear. Other biofuels such as biodiesel have struggled to gain market traction for similar reasons. The ideal renewable fossil fuel replacement requires a feedstock that can be produced and processed with few energy inputs.

