4.3 Properties of the Hydrocarbons
Hydrocarbons are nonpolar substances, with weak intermolecular forces. Their properties are influenced by the lack of strong intermolecular attractive forces. As a group they have relatively low melting and boiling temperatures, and they are poorly or not at all soluble in polar solvents, including water.
The sources of these substances for industry and commerce tend to be geologic deposits of gases (‘natural gas’) and the liquid oil, ‘crude oil.’ These substances along with the solid bitumen make up ‘petroleum,’ a word which in casual use is often used to describe just liquid material. These raw materials are processed in many ways, typically first by distillation to separate out the hydrocarbon components in them. Hydrocarbons are separated by boiling points in distillation, which corresponds to their molecular weights. Fractions containing mixtures of similarly-boiling substances are collected from the distillations, and then can be further refined and/or transformed into products of increasing value.
The lower molecular weight, small hydrocarbons are gases under normal conditions. Many are important substances industrially and in daily life. Methane, for instance, is the principle component of natural gas. It is a gaseous substance with a low boiling point and low reactivity except for its tendency to combust. The slightly larger alkanes ethane, propane and butane are also gases under normal environmental conditions. These also are important industrial feedstocks, and are often combusted to release energy.
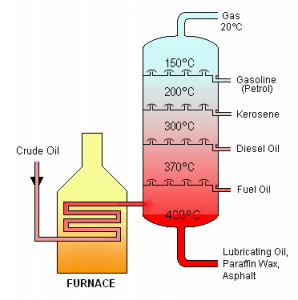
With increased molecular size, the weak London forces operating between alkane molecules begin to provide enough intermolecular attraction for these substances to exist as liquids. Gasoline, for instance, is a complex mixture but consists mostly of alkanes with 5-8 carbons.
Somewhat larger hydrocarbons, while still liquid, are more viscous and have higher boiling points, and are used as kerosene and motor oil. Mineral oil (‘baby oil’) is also composed of alkanes in this group; petroleum jelly (Vaseline™) is similar.
At higher molecular weights, another fraction of alkanes are waxy solids. Blends of this distilled fraction of crude oil are sold as paraffin.
Note that all of the substances described here are obtained through mining: from the tapping of natural gas (methane) wells to the processing of liquid crude oil. These substances originated as dead material from living organisms that have been held and pressurized under the surface of the earth for geologic time spans. In these processes which occur in oxygen-poor environments, the production of hydrocarbons is favored over more oxidized organic compounds like alcohols.
The hydrocarbons are the raw materials used by enormous industries around the world. This includes the fossil fuel and gasoline industry, the plastics industry and the pharmaceutical and cosmetics industries, all of which make heavy use of oil and gas-derived materials. Our quality of life has been heavily impacted by these substances for better and for worse. At the moment these substances are inexpensive and readily-available materials that can be chemically altered to suit our needs, and we rely on them. Substituting for these materials is often difficult because there is no source of hydrocarbons as abundant, anywhere on earth. The geologic processes leading to the formation of petroleum occur in an oxygen-deficient environment, producing highly reduced carbons in these substances from decaying material. At the earth’s surface, abundant oxygen leads to the production of compounds with more oxidized carbons.
The nonpolar nature of crude oil chemicals contributes to environmental problems associated with oil spills. Spilled crude oil floats at the surface of water. It streams outward over huge areas and thus impacts large areas near a spill. Nonpolar materials are not easily removed by water, since they do not dissolve in polar solvents, complicating cleanup.
Alkenes, alkynes and aromatic compounds exist in the complex mixtures we get from oil and gas, but these functional groups also show up in biological material at the surface of the earth. Alkenes and alkynes are prone to some reactions however, which means that they do not exist in stable forms in the environment to the same degree as alkanes.
Physical Properties of Some Selected Alkenes. Like alkanes they have low melting and boiling point temperatures.
| IUPAC Name | Molecular Formula | Condensed Structural Formula | Melting Point (°C) | Boiling Point (°C) |
|---|---|---|---|---|
| ethene | C2H4 | CH2=CH2 | –169 | –104 |
| propene | C3H6 | CH2=CHCH3 | –185 | –47 |
| 1-butene | C4H8 | CH2=CHCH2CH3 | –185 | –6 |
| 1-pentene | C5H10 | CH2=CH(CH2)2CH3 | –138 | 30 |
| 1-hexene | C6H12 | CH2=CH(CH2)3CH3 | –140 | 63 |
| 1-heptene | C7H14 | CH2=CH(CH2)4CH3 | –119 | 94 |
| 1-octene | C8H16 | CH2=CH(CH2)5CH3 | –102 | 121 |
Synthetic Polymers
The most important commercial use of alkenes relates to polymerizations, reactions in which small molecules, referred to in general as monomers, are converted into enormous ones. These polymers are giant molecules formed by the combination of monomers in a repeating manner. A polymer is as different in characteristics from its monomer as a long strand of spaghetti is from a tiny speck of flour. For example, polyethylene, the familiar waxy material used to make plastic bags, is made from the monomer ethylene—a gas. Polyethylene is produced in astounding quantities: as of 2017, over 100 million tons were produced each year (source: Wikipedia).
The Production of Polyethylene
Polyethylene pellets are produced through the polymerization of gaseous ethene to produce the solid product. These pellets, called nurdles, are a commercial product which can be manipulated to form a wide variety of consumer products.
Nurdles of polyethylene can be melted and formed into sheets, bags, bottles, pipes, etc.. It can be mixed with other materials to manipulate the properties of the finished product so that it is harder or more flexible, colored or resistant to ultraviolet light. This versatility has made it immensely popular as a raw material.
Polyethylene is made by a chemical process called addition polymerization, in which monomers add to one another to produce a polymeric product that contains all the atoms of the starting monomers. Ethene molecules are joined together in long chains. The polymerization can be represented by the reaction of a few monomer units:
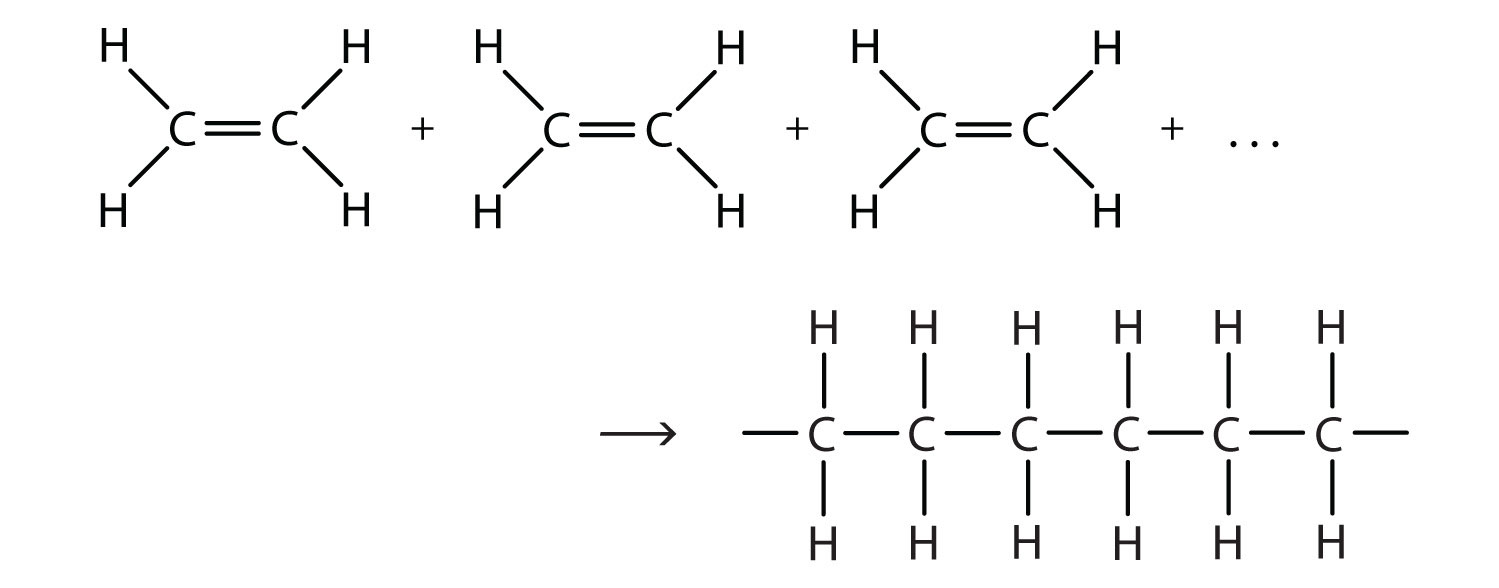
The bond lines extending at the ends in the formula of the product indicate that the structure extends for many units in each direction. Notice that all the atoms—two carbon atoms and four hydrogen atoms—of each monomer molecule are incorporated into the polymer structure.
Polyethylene is so stable that it exists in the environment for a very long time after disposal. With enormous production and such a long lifespan, plastic pollution (from polyethylene as well as other plastics) is increasingly concerning. The discovery of microplastics, which are minute bits of plastic material, in the environment in all kinds of remote environments and in the bodies of animals has led to increasingly worry about the impacts of our plastic use on our health and the health of the environment.
The scale of the problem is immense: More than half the compounds produced by the chemical industry are synthetic polymers. Most of this material resists chemical decomposition through natural processes.
Polyethylene is not the only polymer produced at such a scale. Some other common addition polymers are listed in the table below. Many polymers are mundane (e.g., plastic bags, food wrap, toys, and tableware), but there are also polymers that conduct electricity, have amazing adhesive properties, or are stronger than steel but much lighter in weight. Specialized polymers are increasingly developed and selected for very specific purposes, such as medical applications.
Some Addition Polymers
| Monomer | Polymer | Polymer Name | Some Uses |
|---|---|---|---|
| CH2=CH2 | ~CH2CH2CH2CH2CH2CH2~ | polyethylene | plastic bags, bottles, toys, electrical insulation |
| CH2=CHCH3 |
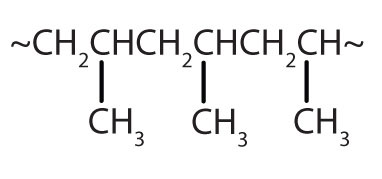 |
polypropylene | carpeting, bottles, luggage, exercise clothing |
| CH2=CHCl |
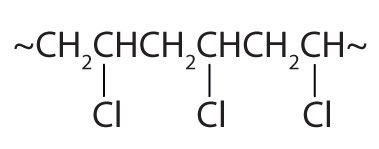 |
polyvinyl chloride | bags for intravenous solutions, pipes, tubing, floor coverings |
| CF2=CF2 | ~CF2CF2CF2CF2CF2CF2~ | polytetrafluoroethylene | nonstick coatings, electrical insulation |
Many natural materials—such as proteins, cellulose and starch, and complex silicate minerals—are also polymers. But as natural products they tend to be subject to natural decay processes, breaking down chemically and by the action of microorganisms in the environment.
Exercise 4.3.1
A substance with molecular structure composed of a large number of (usually) repeating smaller units, called monomers.

