8.3 Addition Reactions
Perhaps the simplest addition reaction is hydrogenation. This is a reaction in which hydrogen gas reacts at a carbon-to-carbon double or triple bond or a carbon-to-oxygen double bond to add hydrogen atoms to carbon atoms. In the laboratory this reaction can be facilitated with hydrogen (H2) in the presence of a catalyst such as nickel (Ni) or platinum (Pt).
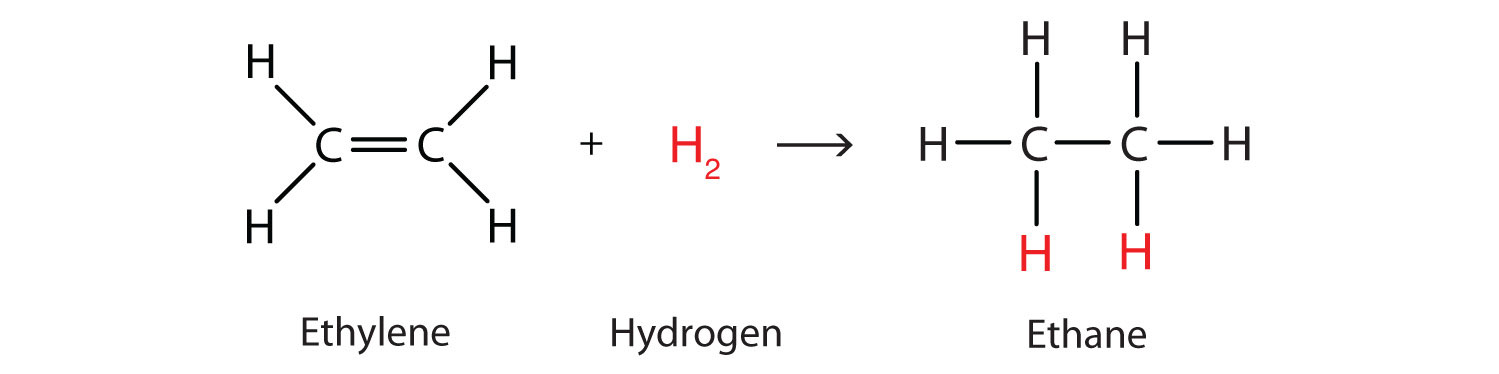
Note that the name ‘addition’ is apt here, since we take a reactant (ethene in this case) and add hydrogen to it, producing a single product (ethane). The carbon skeleton of the reactant is unchanged.
In one important application of this chemistry, hydrogenation is used to convert unsaturated vegetable oils to saturated fats. Hydrogenated fats, or partially-hydrogenated fats often appear on nutrition labels for processed foods such as crackers or chips.
Hydrogenating foods in this way has several benefits: it removes a functional group on the fats that could react by oxidation to make products with off flavors. It also raises the melting points of the fats, changing the texture in ways that might be pleasing to consumers. It allows for higher temperature cooking for baked products, which can increase browning and improve flavor.
But there are also big drawbacks to chemical hydrogenation of fats in foods. During the process, cis double bonds can convert to the trans configuration during the reaction. Trans alkenes in fat molecules are associated with negative effects on cardiac health. Since consumers have largely rejected trans fats due to this, companies have shifted most of their hydrogenation to enzyme-catalyzed processes which yield only cis isomers.
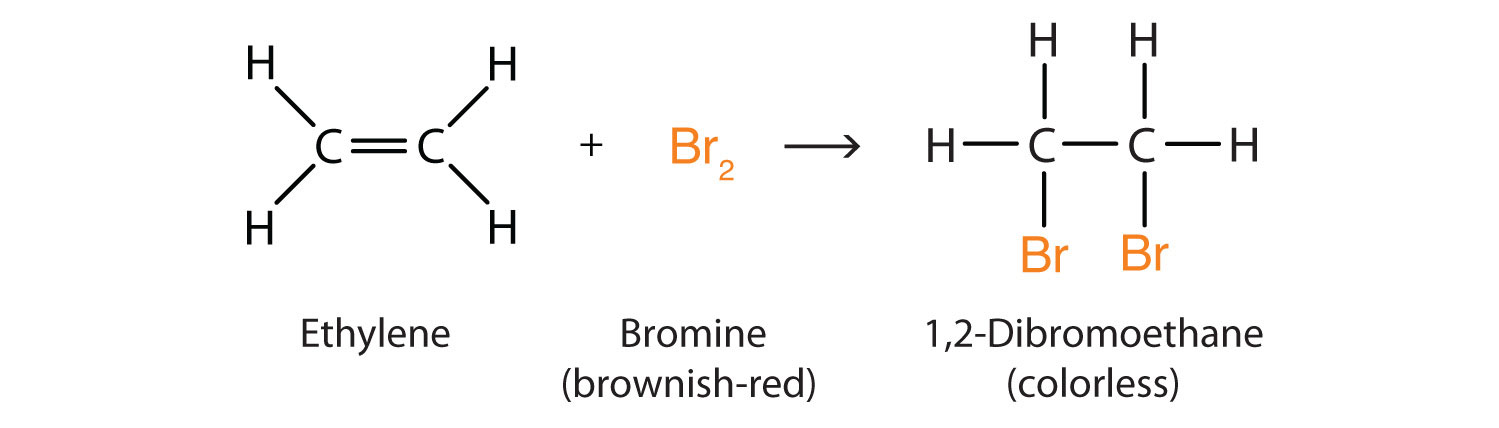
Alkenes also readily undergo halogenation through addition, a reaction in which a halogen reacts at a carbon-to-carbon double or triple bond to add halogen atoms to carbon atoms. Indeed, the addition reaction with bromine (Br2) can be used to test for alkenes in an uncharacterized sample of material. Bromine solutions are brownish red. When we add a Br2 solution to an alkene, the color of the solution disappears because the alkene reacts with the molecular bromine (Br2):
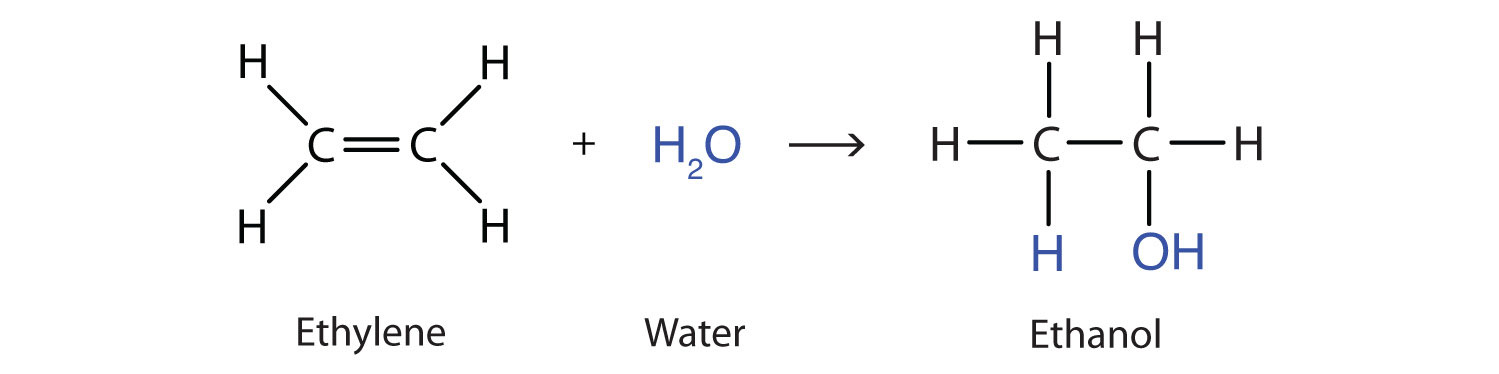
Another important addition reaction occurs between an alkene and water to form an alcohol. This reaction is called hydration, and represents the addition of water to a substance.
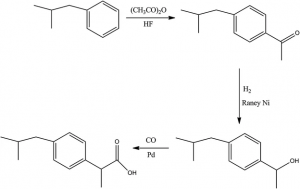
The practice of organic chemistry includes understanding, planning for and carrying out functional group transformations of these types. A knowledgeable organic chemist can link together reactions in order to build specific target molecules according to a plan. Organic chemical synthesis has applications in many industries, from food to pharmaceuticals, adhesives and coatings, and more.