9.2 Properties of Halogenated Compounds
Halogenated Compounds have Varying Polarity
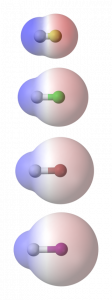
The electronegativity of halogens decreases from fluorine down the group, toward iodine. As a result the bonds between these elements and others vary in their polarity. The image at right shows computer generated electrostatic potential maps of a series of compounds formed between hydrogen and each of the halogens: Fluorine at the top, then Chlorine, Bromine, and finally Iodine.
The atomic sizes vary, so the H-F molecule appears quite small compared to H-I. Also notice the intensity of the translucent red and blue regions. These represent the electron clouds surrounding the nuclei, and the degree to which partial charges exist. The more intensely red a region the more electron-rich and negative the region; the more blue, the more electron-deficient and partially-positive the region. H-F has the most highly polarized of these bonds, with the brightest areas of red and blue color in the computer images.
Bonds between these halogen atoms and carbon would show a similar pattern, with the most polarized bonds occurring between carbon and fluorine. Molecules containing carbon to halogen bonds will thus have varied dipoles. In cases where fluorine or chlorine are present the molecular dipole moments can be quite large, altering the solubility of a compound in water, and affecting its melting and boiling points dramatically. In other arrangements the molecular dipole may be less, either due to less polar bonds to halogens or the arrangement of those atoms in the larger molecule.
For drug developers working with halogenated compounds, these differences are important.
Pharmaceutical compounds travel through the body very differently depending on their water solubility. That solubility relates to the molecular dipole moment. So the addition of halogens to these substances can alter the way a drug is absorbed and transported through a body. This is called the bioavailability of a drug. The best molecule is not worth much if it is unable to get to the organ or tissue where it needs to work.
Drug companies know this and can experiment with alternative structures in order to improve bioavailability. Sometimes they can alter existing, somewhat effective drugs in their search for better alternatives. But they also need to consider the breakdown and excretion of these substances as well, which is also be affected by changes to water-solubility. None of the work is simple, but it is important. A whole area called pharmacokinetics is focused on the movement of drugs through the body.

