7.3 Carboxylic Acids
Carboxylic Acids
The carboxylic acid functional group includes a carbonyl carbon connected directly to an alcohol group, as shown below. Carboxylic acids are common in nature. Their properties are quite different from the other carbonyl compounds so far mentioned. Notably, as the name suggests, carboxylic acids are acidic.
Many carboxylic acids are known by their common names, including lactic acid, citric acid, glutamic acid, and others. Systematic names for these substances also exist. They are modifications of the name of the corresponding hydrocarbons to include the suffix ‘-oic acid.’ For instance:
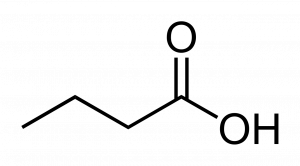
This structure is butanoic acid.
As with some other organic families, common names are very frequently used with carboxylic acids and some are similar to, but not quite aligned with IUPAC rules. This is a difficulty for students, but sometimes enough information from the IUPAC system is included in a common name to allow you to decipher the structure.
To illustrate, the carboxylic acid above is often called by its common name ‘butyric acid.’ Butyric comes from the ancient Greek word for butter, the source from which this compound was first isolated. Butyric acid is a breakdown product of the fats in milk that actually is associated with spoilage in butter and cheeses, and while it contributes to flavor in some foods (e.g. Parmesan cheese) also occurs in stale perspiration and vomit!

Similar to the situation for aldehydes, the carboxylic acid group always exists at the end of the parent chain because the functional group requires 3 of 4 available valence electrons on the carbonyl carbon. The carbonyl carbon in these molecules is designated as carbon 1, so location signifiers are not required to pinpoint the location of a carboxylic acid group, unless a name is describing a complex substance with more than one of these groups on it.
Both oxygen atoms on the carbonyl carbon in a carboxylic acid are electronegative, drawing electron density away from surrounding atoms toward themselves. The hydrogen atom is acidic, due to the stability of the anion that exists when that hydrogen ion dissociates from the larger structure.

Carboxylic acids in water solution exist in an equilibrium between the acid and conjugate base states, shown here as the reactant and the anion product of this reaction. The double-headed arrow shown in the figure indicates the nature of this reaction is an equilibrium, with both forward and reverse reactions occurring to some degree. Carboxylic acids tend to be mostly in the reactant form at physiologic pH, with perhaps 5% of the substance in a deprotonated, anion form at any given moment.
This amount of acid added to a solution from this type of dissociation of a carboxylic acid, however, is significant in many contexts. For instance the acidity of ethanoic acid, also known as acetic acid, gives vinegar solutions their sour taste and contributes to their ability to retard the growth of undesirable organisms in pickles. Lactic acid in yogurt is another example of a carboxylic acid that has a big influence on its medium, as milk is converted by lactic-acid forming bacteria to kefir or yogurt.
Exercise 7.3.1

All of the substances in the interactive questions below are present in many ripe cheeses, including the famously funky cheese named Camembert.
Exercise 7.3.2

