2.4 Heteroatoms and Functional Groups
Functional groups in organic compounds
Functional groups are structural units within organic compounds that are defined by specific bonding arrangements between specific atoms. Many, but not all, functional groups contain heteroatoms: atoms other than carbon and hydrogen. The structure below of capsaicin, the heat-sensation producing molecule in hot peppers, incorporates several functional groups, labeled in the figure and explained throughout this section.
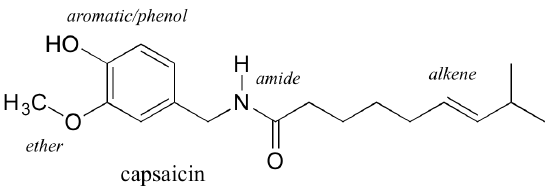
Functional groups are the key structural elements that define how organic molecules act. Our focus for now will be on drawing and recognizing each functional group, as depicted by structural formulas and line-bond structures. But as is implied by the name, functional groups are linked to the behavior of substances, their impact on properties of physical and chemical properties of substances. As the structural feature of a wing on an animal is associated with its ability to fly, functional groups on molecules are structural features that are associated with what those substances can do.
This section includes a quick tour through a collection of functional groups. You are not expected to know all of the details completely after one reading. The overview approach can help you appreciate the variety of structures within organic chemistry, and can help you begin to build a vocabulary. Later we will slow down and go through many of the most important of these groups again, in more detail.
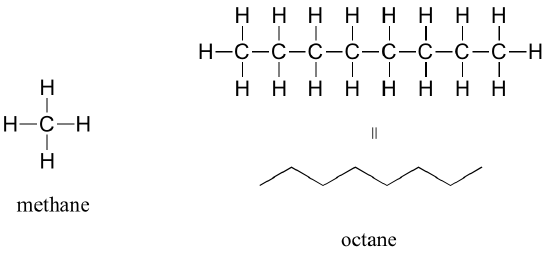
The ‘default’ in organic chemistry (essentially, the lack of any functional groups) is described as alkane, characterized by single bonds between carbon and carbon, or between carbon and hydrogen. Methane, CH4, is an alkane that is the combustible natural gas you may burn in your furnace to heat your home. Octane, C8H18, is an alkane which is a component of gasoline.
Alkenes (sometimes called olefins) have carbon-carbon double bonds, and alkynes have carbon-carbon triple bonds. Ethene is a gaseous alkene that serves as a cellular signal in fruits to stimulate ripening. Fruits that are sensitive to this signaling molecule can be placed in a paper bag along with an apple – the apple emits ethene gas, setting off the ripening process in the fruit. Commercial fruit packers can make use of this phenomenon by harvesting unripe fruits, then inducing ripening right before shipping to consumers. Ethyne, commonly called acetylene, is an alkyne used as a fuel in welding blow torches.
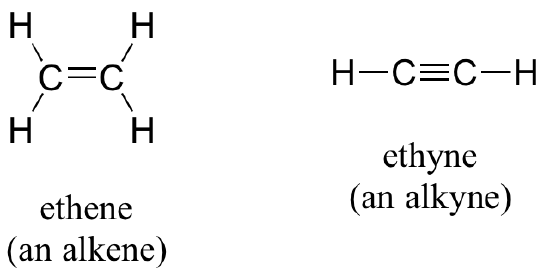
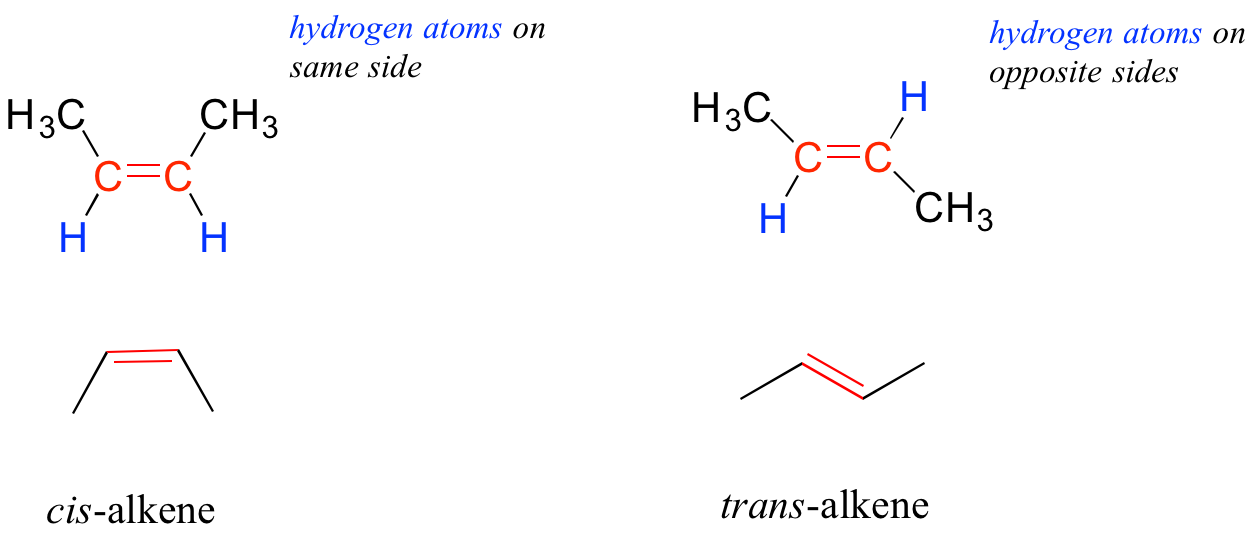
The bonding in alkenes is trigonal planar and the molecules are unable to rotate along the axis of the bond. The double bonds thus lead to 120 degree bond angles and a planar triangular geometry around the double bond. They are locked into one of two geometric configurations. These forms are given the descriptive names: cis or trans. The cis and trans alkenes below are different molecules with different physical properties.
In alkynes the geometry around the triple bond is linear (bond angles are 180º) and only one other atom can bond to the alkyne carbon, so while there is no rotation along the axis of the triple bond, such geometric isomers do not exist for this group.
Alkanes, alkenes, and alkynes are all classified as hydrocarbons, because they are composed solely of carbon and hydrogen atoms. Alkanes are said to be saturated hydrocarbons, because the carbons are bonded to the maximum possible number of hydrogens – in other words, they are saturated with hydrogen atoms. The double and triple-bonded carbons in alkenes and alkynes have fewer hydrogen atoms bonded to them – they are thus referred to as unsaturated hydrocarbons.
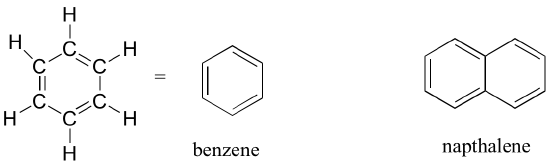
The aromatic group is exemplified by benzene and naphthalene. Aromatic groups are planar (flat) ring structures, and are widespread in nature so you will see them frequently if you encounter chemical structures in biology classes or in biomedical work.
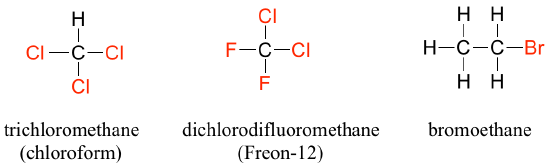
When the carbon of an alkane is bonded to one or more halogens, the resulting compound is called an alkyl halide or haloalkane. For many years chloroform, a haloalkane with the formula CHCl3 was a commonly used solvent in the laboratory. This substance was also one of the earlier anesthetic drugs used in surgery. It’s use is now highly restricted due to negative health effects, but it remains an important industrial chemical used in the production of PTFE (TeflonTM). Chlorodifluoromethane was used as a refrigerant and in aerosol sprays until the late twentieth century, but its use was discontinued after it was found to have harmful effects on the ozone layer. Bromoethane is a simple alkyl halide often used in organic synthesis. Alkyl halides groups are quite rare in biomolecules.
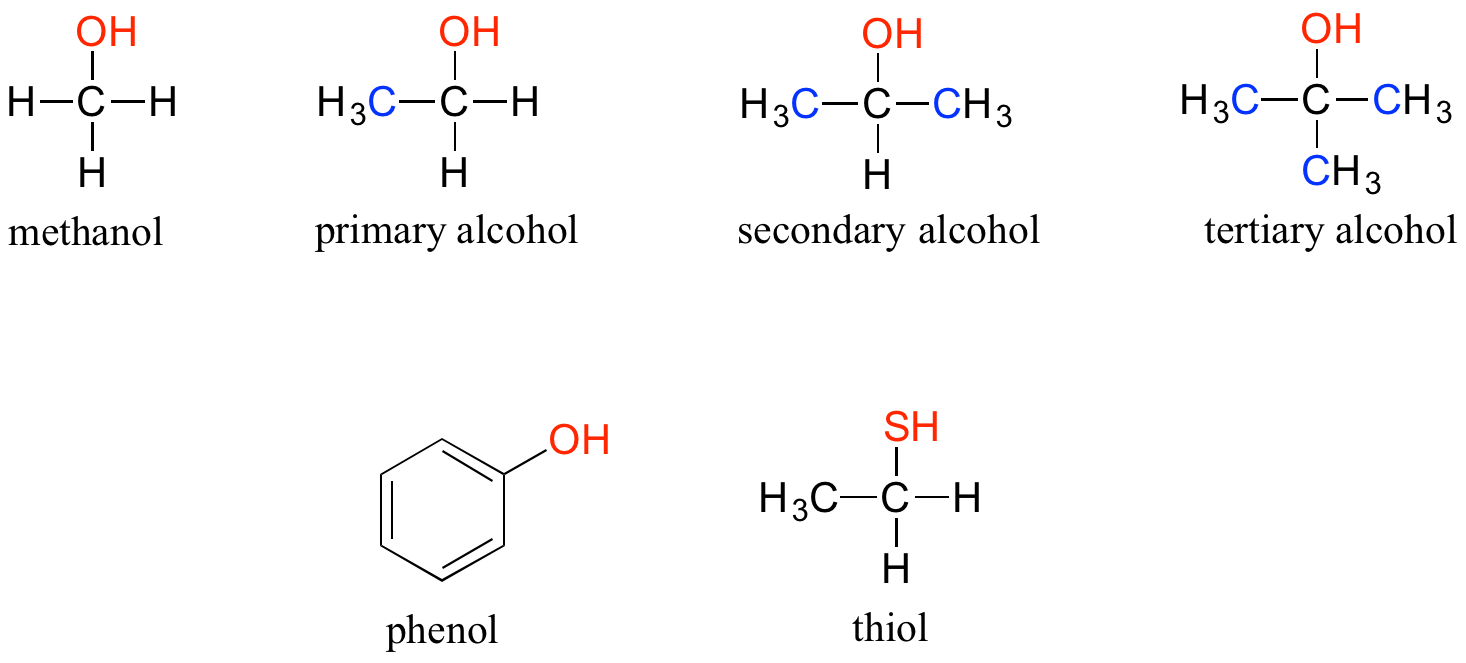
In the alcohol functional group, a carbon is single-bonded to an OH group (the OH group, by itself, is referred to as a hydroxyl). Except for methanol, all alcohols can be classified as primary, secondary, or tertiary. When the hydroxyl group is directly attached to an aromatic ring, the resulting group is called a phenol. The sulfur analog of an alcohol is called a thiol (from the Greek thio, for sulfur).
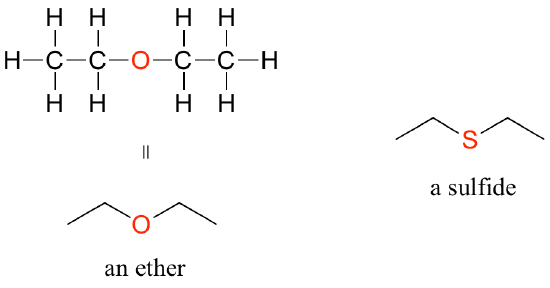
In an ether functional group, a central oxygen is bonded to two carbons. Below is the structure of diethyl ether, a common laboratory solvent and also one of the first compounds to be used as an anesthetic during operations. The sulfur analog of an ether is called a thioether or sulfide.
Amines are characterized by nitrogen atoms with single bonds to hydrogen and carbon. Just as there are primary, secondary, and tertiary alcohols, there are primary, secondary, and tertiary amines. Ammonia is a special case with no carbon atoms.
There are a number of functional groups that contain a carbon-oxygen double bond, which is commonly referred to as a carbonyl. Ketones and aldehydes are two closely related carbonyl-based functional groups that react in very similar ways. In a ketone, the carbon atom of a carbonyl is bonded to two other carbons. In an aldehyde, the carbonyl carbon is bonded on one side to a hydrogen, and on the other side to a carbon. The exception to this definition is formaldehyde, in which the carbonyl carbon has bonds to two hydrogens.
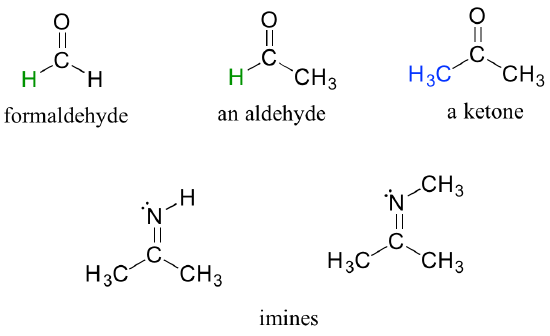
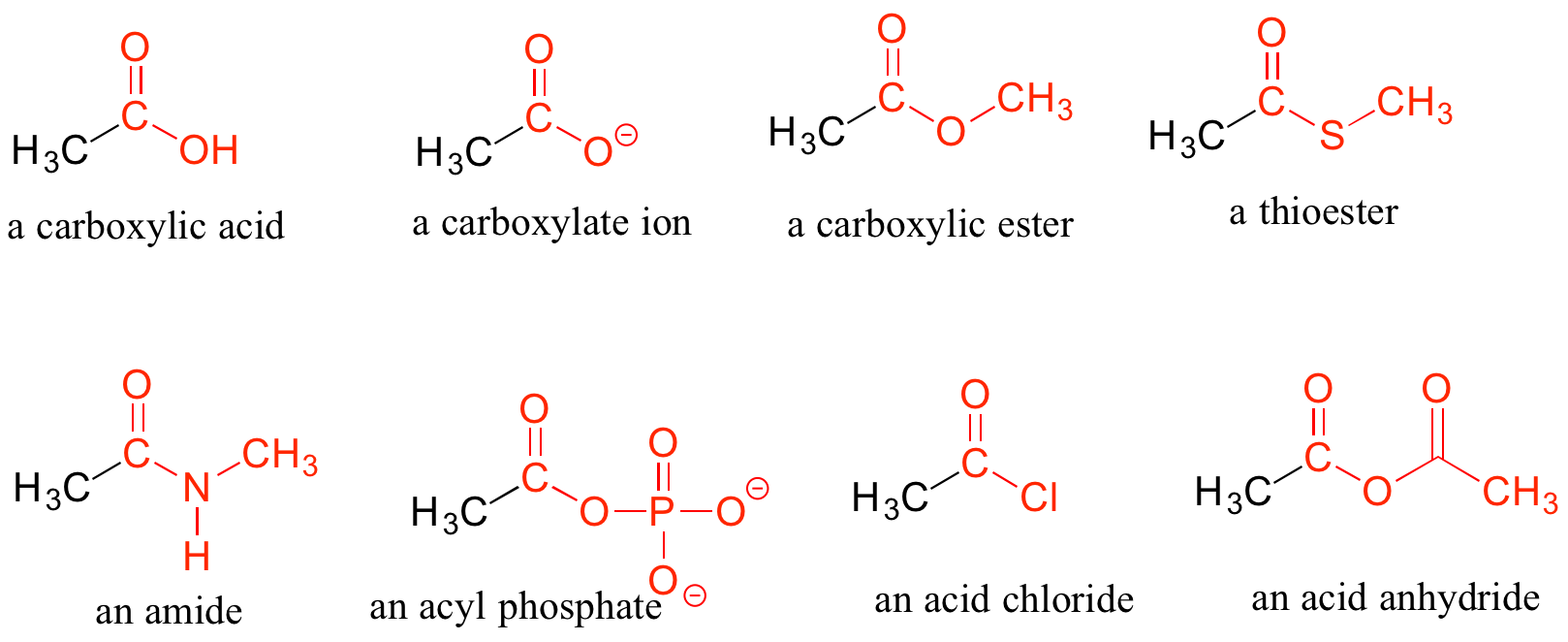
When a carbonyl carbon is bonded on one side to a carbon (or hydrogen) and on the other side to an oxygen, nitrogen, or sulfur, the functional group is considered to be one of the ‘carboxylic acid derivatives’, a designation that describes a set of related functional groups. The eponymous member of this family is the carboxylic acid functional group, in which the carbonyl is bonded to a hydroxyl group. Other derivatives are carboxylic esters (usually just called ‘esters’) and amides. Other carboxylic acid derivatives also exist. Many are common in biology.
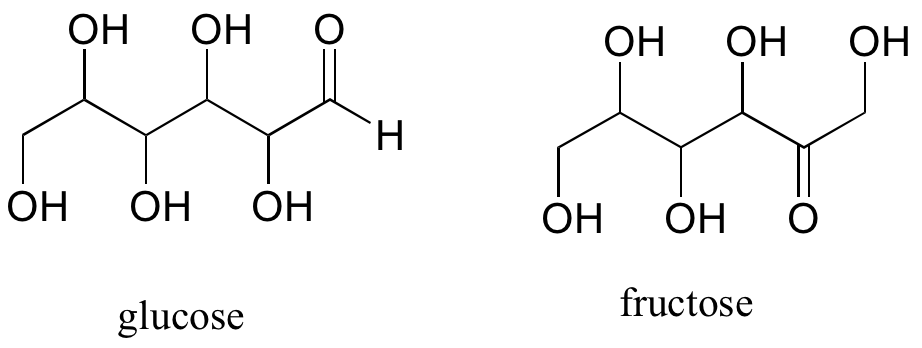
A single compound often contains several functional groups, particularly in biological organic chemistry. The six-carbon sugar molecules glucose and fructose, for example, contain aldehyde and ketone groups, respectively, and both contain five alcohol groups.
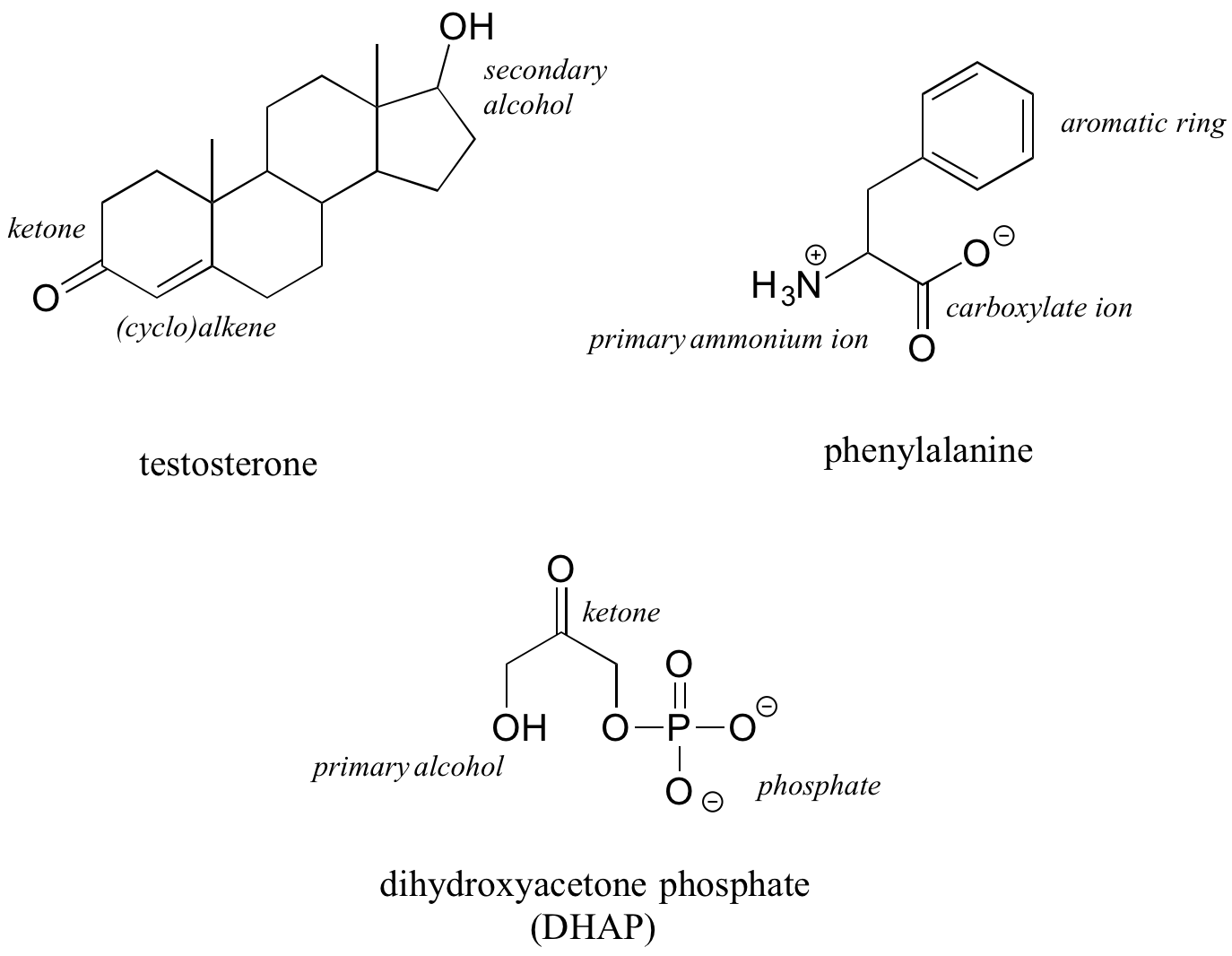
The hormone testosterone, the amino acid phenylalanine, and the glycolysis metabolite dihydroxyacetone phosphate all contain multiple functional groups, as labeled below.
While not in any way a complete list, this section has covered most of the important functional groups that we will encounter in biological organic chemistry.
Exercise 2.4.1
Exercise 2.4.2
Attempt to identify the functional groups (other than alkanes) in the following organic compounds. If you find alcohol or amine groups, identify them as primary, secondary, or tertiary.
a) 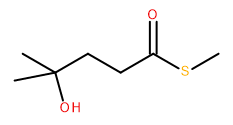 b)
b) 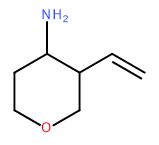
c) 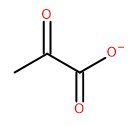 d)
d) 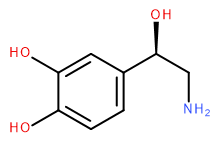
Exercise 2.4.3
As an add-on to the exercise above, write out a short explanation for how you went about identifying the secondary alcohol. What did you look at, or count, to figure this out?
Exercise 2.4.4
Draw one example each of compounds fitting the descriptions below, using line structures. If you can’t draw them, describe them with words. Be sure to designate the location of all non-zero formal charges. All atoms should have complete octets (phosphorus may exceed the octet rule). There are many possible correct answers for these, so be sure to check your structures with your instructor.
a) a compound with molecular formula C6H11NO that includes alkene, secondary amine, and primary alcohol functional groups
b) a molecule that includes aldehyde, secondary alcohol, and phosphate functional groups.
c) A compound with molecular formula C6H9NO that has an amide functional group, and does not have an alkene group.
Functional Groups and Organic Nomenclature
As noted earlier, the presence of a functional group frequently shows up in the IUPAC name as a suffix. For alkanes, the names end in ‘ane,’ which indicates the absence of any functional group.
Alkenes are designated with an ‘ene’ ending, and when necessary the location and geometry of the double bond are indicated. Compounds with multiple double bonds are called dienes, trienes, etc.
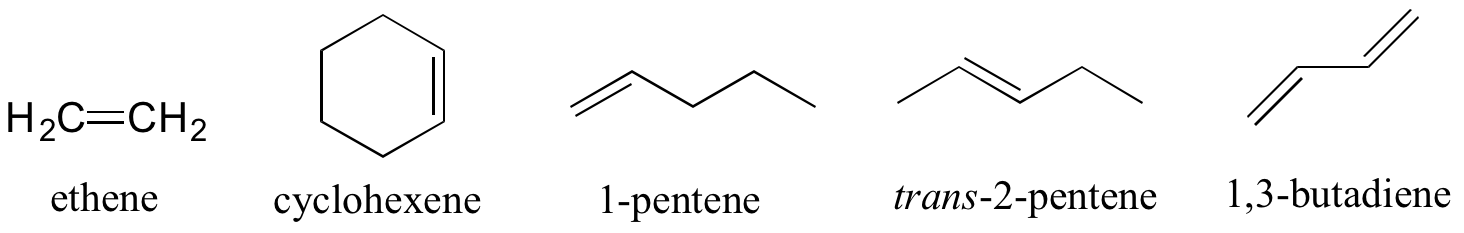
Some groups can only be present on a terminal carbon, and thus a locating number is not necessary: aldehydes end in ‘al’, carboxylic acids in ‘oic acid’, and their conjugate base carboxylates in ‘oate’.
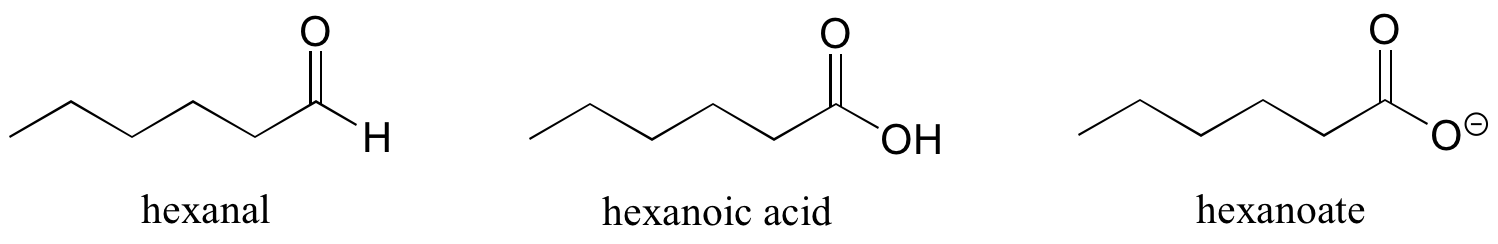
Other functional groups have their suffixes, as well, and some functional groups affect IUPAC names in more complex ways. Many molecules also have multiple functional groups on them, complicating the names further.
It is not crucial to learn the details now, but it is valuable to know that the suffix can often be used to identify the presence of a specific functional group on a molecule.
Exercise 2.4.5-9
Drawing abbreviated organic structures
Often when drawing organic structures, chemists find it convenient to use the letter ‘R’ to designate part of a molecule outside of the region of interest. If we just want to refer in general to a functional group without drawing a specific molecule, for example, we can use ‘R groups’ to focus attention on the group of interest:
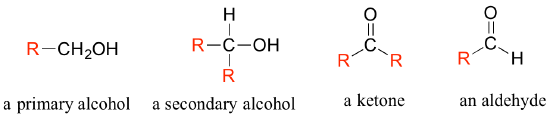
The ‘R’ group is a convenient way to abbreviate the structures of molecules, especially when we are interested in something that is occurring specifically at one location on the molecule. For example, when considering the oxidation and reduction of the biologically-important flavin molecule, abbreviating the flavin structure helps a reader focus on the most important part of the molecule:
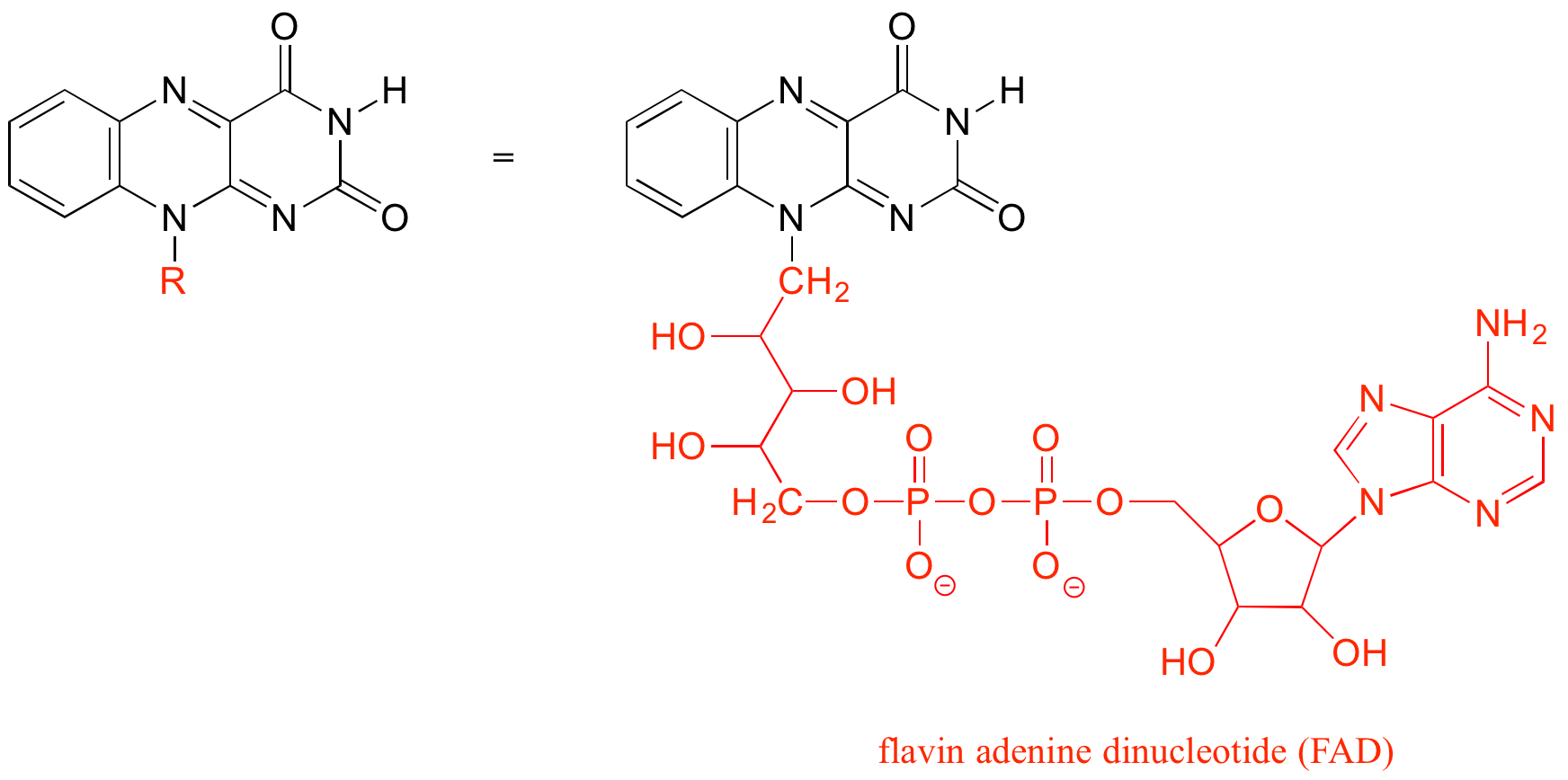
As an alternative, we can use a ‘break’ symbol to indicate that we are looking at a small piece or section of a larger molecule. This is used commonly in the context of drawing groups on large polymers such as proteins or DNA.

Finally, ‘R’ groups can be used to concisely illustrate a series of related compounds, such as the family of penicillin-based antibiotics.
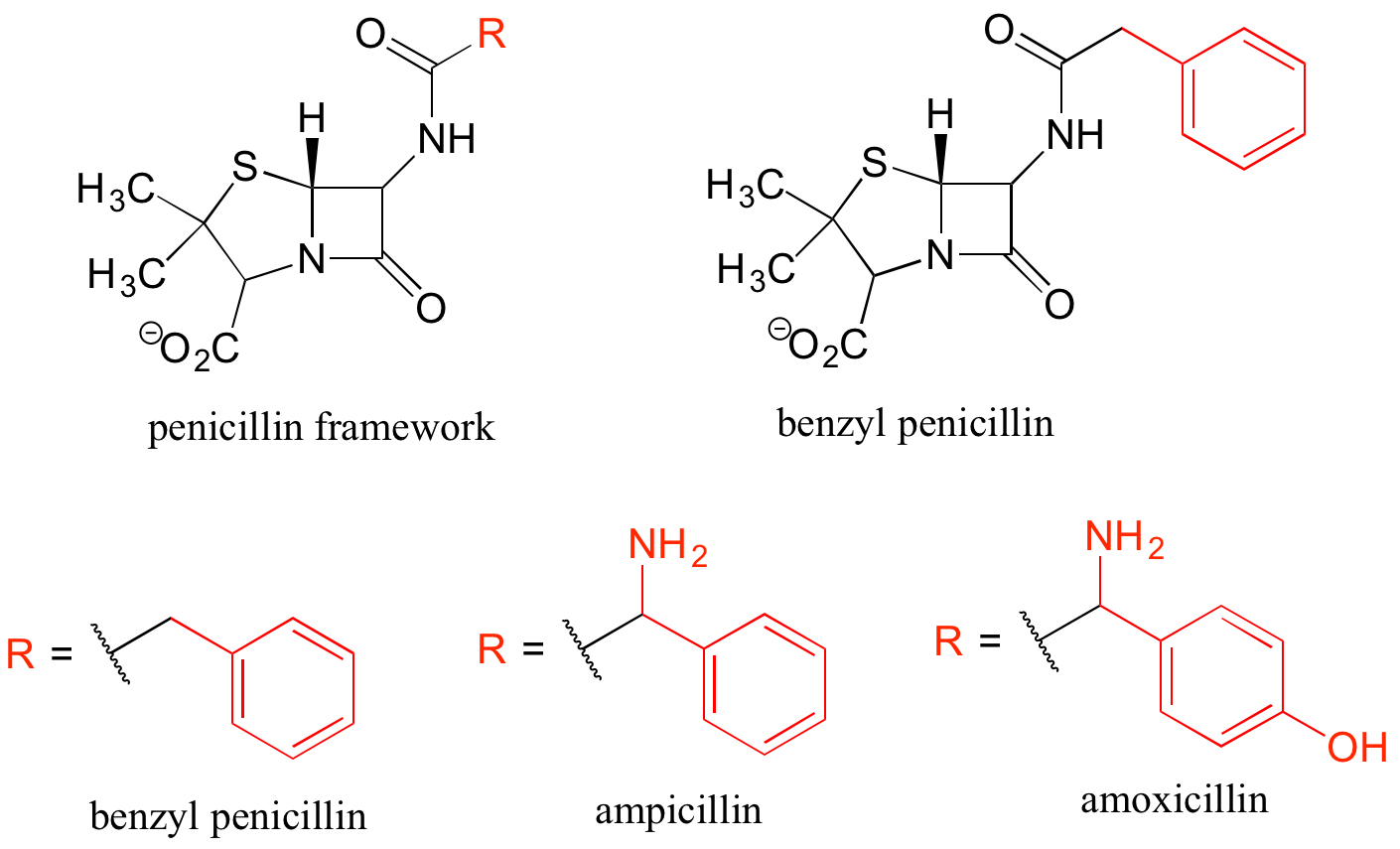
Using abbreviations appropriately is very helpful to students interested in biology, because although many biomolecules are very large and complex (and take forever to draw!), usually we are focusing on just one small part of the molecule where a change is taking place. Abbreviations show up frequently in that context.
geometry around a central atom which leads to 120 degree bond angles. Planar indicates "flat," which describes the overall 4 atom structure (the central atom and 3 connected atoms), which all exist on a common plane.

