3.3 Melting points and Boiling Points
Boiling point and melting point
The observable melting and boiling points of different organic molecules provides an additional illustration of the effects of noncovalent interactions. The overarching principle involved is simple: how strong are intermolecular forces within a pure sample of the substance? Melting and boiling are processes in which these noncovalent attractive interactions are disrupted. The stronger the intermolecular attractive forces, the more energy that is required to break them apart. That energy is in the form of heat, e.g. a higher temperature must be reached for the melting or boiling to occur.
As a rule, larger molecules have higher boiling and melting points. Consider the boiling points of increasingly larger hydrocarbons. More carbons and hydrogens create a greater surface area possible for London forces, and thus higher boiling points. Below zero degrees Celsius (and at atmospheric pressure) butane is a liquid, because the butane molecules are held together by these forces. Above zero degrees, however, the molecules gain enough thermal energy to separate from one another and enter the gas phase. Octane, in contrast, remains in the liquid phase all the way up to 128oC, due to the increased influence of attractive London forces, made possible by the larger surface area of the individual molecules.
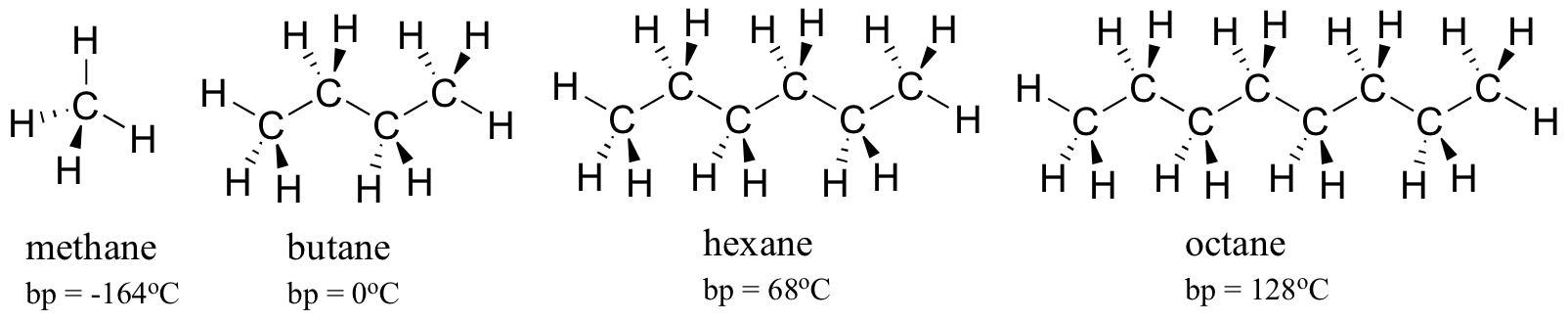
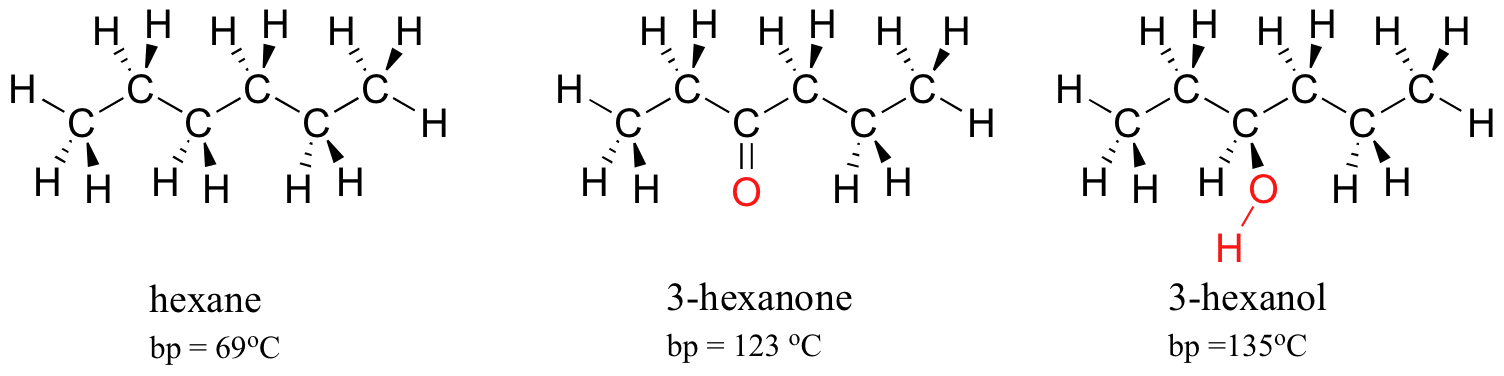
The strength of intermolecular hydrogen bonding and dipole-dipole interactions is reflected in higher boiling points. Look at the trend for hexane (London forces only), 3-hexanone (dipole-dipole interactions), and 3-hexanol (hydrogen bonding). In all three molecules, London forces are significant. The polar ketone group allows 3-hexanone to form intermolecular dipole-dipole interactions, in addition to the weaker London forces. 3-hexanol, because of its hydroxyl group, is able to form intermolecular hydrogen bonding interactions, which are stronger yet.
Of particular interest to biologists (and pretty much anything else that is alive on the planet) is the effect of hydrogen bonding in water. Because it is able to form tight networks of intermolecular hydrogen bonds, water remains in the liquid phase at temperatures up to 100 OC despite its small size. The world would obviously be a very different place if water boiled at 30 OC.
Exercise 3.3.1
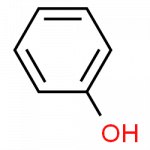
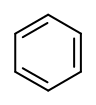
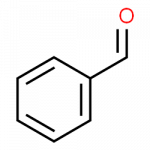
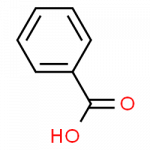
Wikipedia pages for these substances can be found through these links, for phenol, benzene, benzaldehyde and benzoic acid.
- phenol
- benzene
- benzaldehyde
- benzoic acid
By thinking about noncovalent intermolecular interactions, we can also predict relative melting points. All of the same principles apply: stronger intermolecular interactions result in a higher melting point. Ionic compounds, as expected, usually have very high melting points due to the strength of ion-ion interactions. Just like with boiling points, the presence of polar and hydrogen-bonding groups on organic compounds generally leads to higher melting points. The size of a molecule influences its melting point as well as its boiling point, again due to increased van der Waals interactions between molecules.
What is different about melting point trends versus boiling point or solubility trends, is the importance of a molecule’s shape and its ability of pack tightly together. Picture yourself trying to make a stable pile of baseballs on the floor. It just doesn’t work, because spheres don’t pack together well – there is very little area of contact between each ball. It is very easy, though, to make a stack of flat objects like books.
The same concept applies to how well molecules pack together in a solid. The flat shape of aromatic compounds allows them to pack efficiently, and thus aromatics tend to have higher melting points compared to non-planar hydrocarbons with similar molecular weights. Comparing the melting points of benzene and toluene, you can see that the extra methyl group on toluene disrupts the molecule’s ability to pack tightly, thus decreasing the cumulative strength of intermolecular van der Waals forces and lowering the melting point.
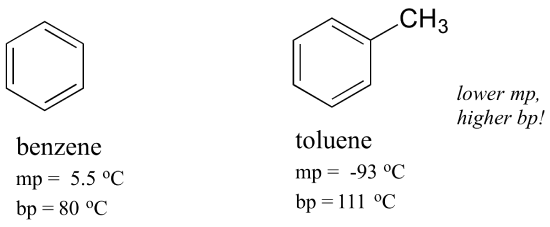
Note also that the boiling point for toluene is significantly above the boiling point of benzene! The key factor for the boiling point trend in this case is size (toluene has one more carbon), whereas for the melting point trend, shape plays a much more important role. This makes sense when you consider that melting involves ‘unpacking’ the molecules from their ordered array, whereas boiling involves simply separating them from their already loose (liquid) association with each other.
Exercise 3.3.3