5.1 Names and Structures for Alcohols, Thiols, Ethers, and Amines
Now that we have learned quite a lot about the hydrocarbons, we are ready to venture into discussion of other organic families. Those described in this chapter include the alcohols, thiols, ethers and amines. Since these families include functional groups that contain heteroatoms, there will be some differences between them and the hydrocarbons. For each of the four groups considered here, as well as for additional organic families we will discuss later, we will organize our learning into several sections.
- identifying the functional group on a structure
- drawing from names, and naming structures
- explaining the physical properties of these substances, including discussion of melting points, boiling points, and solubility in water and other solvents
Interspersed throughout, you will be introduced to a few new ideas that may have specific relevance to the functional group under consideration. Reactivity is also related to organic families, but we are postponing that conversation until a bit later.
Alcohols
An alcohol can be recognized by the presence of the hydroxy or alcohol functional group on an organic molecule. Consider the structure shown below:
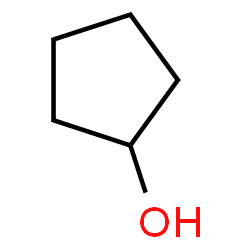
The oxygen and hydrogen connected to a carbon constitute the alcohol functional group. Atoms that are not carbon or hydrogen in organic molecules are often referred to as heteroatoms, where ‘hetero’ indicates ‘other,’ meaning an atom of an element other than carbon or hydrogen. When faced with a large molecule you wish to understand it is smart to first find the heteroatoms. They are indicators of functional groups, and in that role can have a strong influence on the properties of these substances.
Alcohol names are related closely to the names of the hydrocarbons they resemble, with key modifications. For instance, the molecule above without the hydroxy group would be named cyclopentane. The alcohol is named cyclopentanol.
The ‘-ol’ suffix may require a location signifier. When this is the case, provide a number that indicates the number of the carbon in the parent chain where the oxygen is attached. For cyclopentanol this is not necessary because there are not distinct carbons that are numbered. But in this example a number is required:
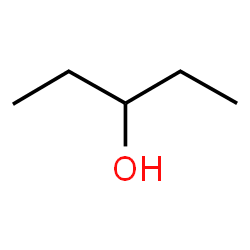 Numbering from either end of the parent chain (5 carbons) would identify carbon 3 as the point where the hydroxy (-OH) group connects to the chain. So the name of this alcohol is 3-pentanol. You may also see the more technically correct (according to IUPAC) pentane-3-ol, but this form of nomenclature has been slow to catch on in practice.
Numbering from either end of the parent chain (5 carbons) would identify carbon 3 as the point where the hydroxy (-OH) group connects to the chain. So the name of this alcohol is 3-pentanol. You may also see the more technically correct (according to IUPAC) pentane-3-ol, but this form of nomenclature has been slow to catch on in practice.
As with branches on an alkane, the number is assigned from whichever end of the parent chain produces the lowest possible number for the alcohol.
Aromatic rings with alcohol groups directly attached to them are different enough than non-aromatic (‘aliphatic’) alcohols to warrant their own names. Compounds containing this structure are named phenols, after the name of the substance below, which is the simplest of these aromatic alcohols, and is named phenol:
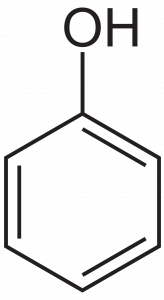
There are of course variations that make for extra challenge: branched hydrocarbon structures with alcohol groups and molecules with more than one functional group, for instance. Those issue can be dealt with later, once you have more experience.
Exercise 5.1.1-2
Thiols
Thiols are substances that are structurally quite similar to alcohols, however they include Sulfur where Oxygen exists in an alcohol. The group -SH can be referred to as a thiol group. Occasionally the common name mercaptan is still used to describe members of this organic family.
Naming thiols involves a modification of the name of the corresponding hydrocarbon. In this case, rather than removing the ‘-ane’ from an alkane name, the suffix changes to ‘-anethiol.’ For instance:

is named 2-propanethiol.
These names will seem odd as you first learn them and you might find pronunciation unclear or difficult. That is normal. The names are designed to first convey structural information and only secondarily to be easy to speak.
Exercise 5.1.3
Ethers
Ethers are organic molecules that also contain oxygen. In this case, the oxygen is incorporated into a chain of carbons, so that it has two single-bond connections to two separate carbons. IUPAC rules for naming ethers involve naming one side of the ether as if it were a substituent group on the other carbons. This is somewhat complicated. Making matters worse for students, the systematic names for ethers are not used consistently by chemists. So common names, which utilize two separate words indicating each side of the ether, predominate:
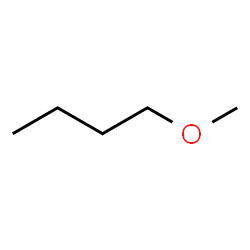 This structure is named by IUPAC rules as Methoxybutane. The ‘methoxy’ portion describes a group that includes one carbon (and its hydrogens) and the oxygen, built onto a butane parent chain. The common name for this structure is methyl butyl ether.
This structure is named by IUPAC rules as Methoxybutane. The ‘methoxy’ portion describes a group that includes one carbon (and its hydrogens) and the oxygen, built onto a butane parent chain. The common name for this structure is methyl butyl ether.
What are students to do? Ask for clarification on the expectations for naming and drawing ether structures. You will be given guidance.
Exercise 5.1.4
Amines
The amine functional group is composed of a nitrogen atom bonded to at least carbon by a single bond. Since nitrogen has 5 valence shell electrons it typically forms 3 bonds to other atoms and has one lone pair of electrons. Nitrogen atoms with one connection to carbon are called primary amines, those with two connections to carbons secondary amines, and those with three connections to carbon are tertiary amines.
The names of primary amines are formed by replacing the ‘-e’ ending on an alkane name with ‘-amine.’ As with other functional groups, the placement on the parent chain is noted with a number. For instance:
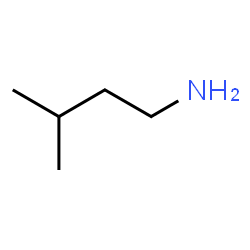
This is 3-methyl-1-butanamine. Note that the numbering is driven by the location of the functional group rather than the branch on the alkane. Functional groups nearly always have priority over other groups when locations are assigned in this way.
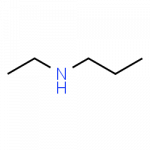
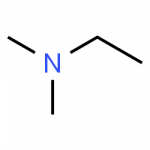
Secondary and tertiary amines, which have additional alkyl (carbon-based) groups attached to the nitrogen, are handled by adding a prefix describing the additional alkyl groups and indicating the location for attachment as ‘N.’ as demonstrated here:
- N-ethylpropanamine
- N,N-dimethylethanamine
Exercise 5.1.5
Recall that hydrogen atoms are not shown in line-bond structures for hydrocarbons. When attached to heteroatoms, however, hydrogens are almost always shown explicitly on the structures.
The collection of rules governing naming is complex, since the structures of organic chemistry are so varied. It is not possible for anyone to learn to name everything, and even those who work as professional chemists for years still encounter situations where it is difficult for them to confidently assign a name to a structure (or a structure to a name). But the existence of the system is helpful, and provides an approach to organizing and making sense of the wide array of organic compounds that exist.