6.2 Stereochemical Designations in Names and Structures
Nomenclature for distinguishing chiral isomers
Chemists need a convenient way to distinguish one stereoisomer from another. The Cahn-Ingold-Prelog system is a set of rules that allows us to unambiguously define the stereochemical configuration of any stereocenter, using the designations ‘R ’ or ‘ S .’ The label ‘R’ comes from the Latin word rectus, suggesting ‘right,’ and is related to the Spanish words “derecha” and “derecho.” S comes from the Latin sinister, which can be translated as ‘left’ (“sinistra” is “left” in Italian).
In an organic structure with chiral carbons, each chiral atom gets one of these designations. So the names for such compounds includes the letters. For instance:
(1S,2S)-2-methylamino-1-phenylpropan-1-ol
is the IUPAC name describing the structure of pseudoephedrine, which includes the designations of two chiral carbons at positions 1 and 2 on the parent chain. This substance is the active ingredient in the decongestant medication SudafedTM.
A step-wise method for accurately assigning an R/S designation to a chiral carbon:
Step 1: Assign priorities to the four substituents attached directly to the carbon. Priorities are based on the atomic number of the atom attached to the chiral carbon. If these are identical (a tie) other rules come into play. The highest priority substituent will be group number 1, others are numbered in decreasing order 2, 3 and 4.
Step 2: Imagine a circle from 1 to 2 to 3.
Step 3: Determine the orientation of the #4 priority group. If it is oriented into the plane of the page (away from you), go to step 4a. If it is oriented out of the plane of the page (toward you) go to step 4b.
Step 4a: a clockwise circle as you move from group 1 to 2 to 3 corresponds to a carbon with the R configuration, while a counterclockwise circle corresponds to a carbon with the S configuration.
Step 4b: a clockwise circle in part 2 corresponds to the S configuration, while a counterclockwise circle corresponds to the R configuration.
We’ll use the 3-carbon sugar glyceraldehyde as an example.
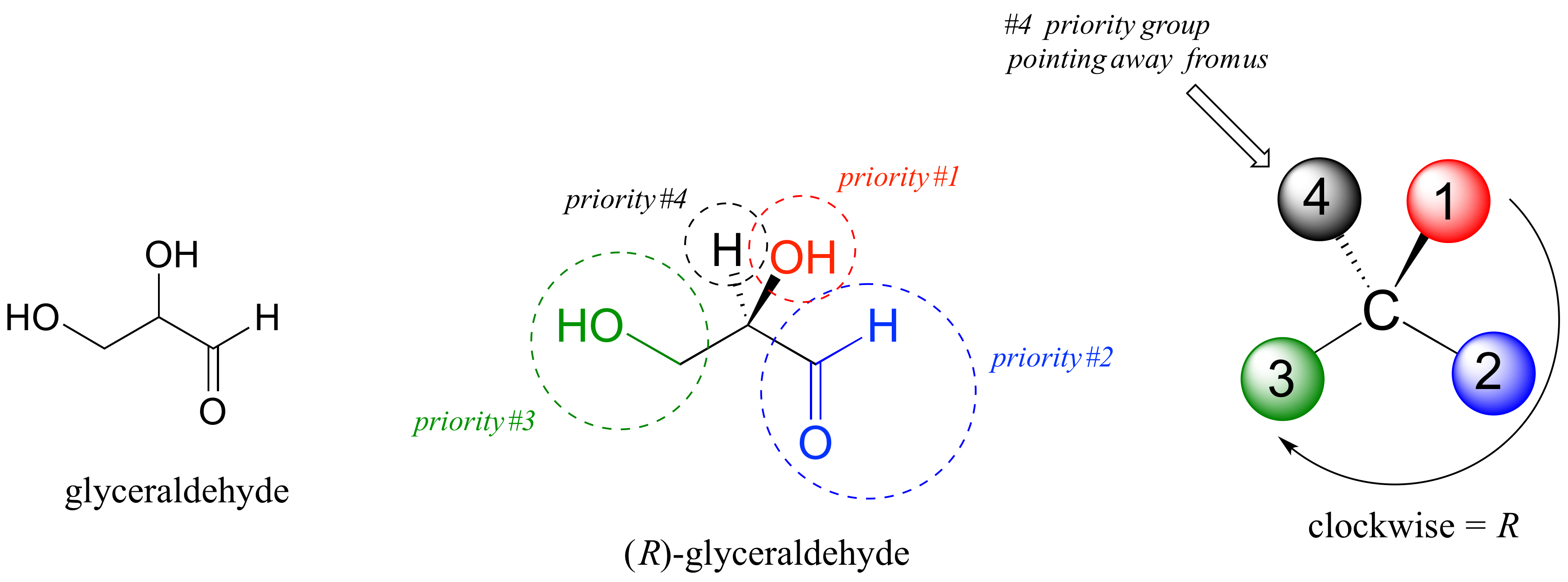
The first thing that we must do is to assign a priority to each of the four substituents bound to the chiral center. We first look at the atoms that are directly bonded to the chiral center: these are H, O (in the hydroxyl), C (in the aldehyde), and C (in the CH2OH group).
Two priorities are easy: based on additional rules of the R/S system, hydrogen, with an atomic number of 1, is considered the lowest (#4) priority, and the hydroxyl oxygen, with atomic number 8, is priority #1. Carbon has an atomic number of 6. Which of the two ‘C’ groups is priority #2, the aldehyde or the CH2OH? To determine this, the system instructs us to move one more bond away from the chiral center: for the aldehyde we have a double bond to an oxygen, while on the CH2OH group we have a single bond to an oxygen. If the atom is the same, double bonds have a higher priority than single bonds. Therefore, the aldehyde group is assigned #2 priority and the CH2OH group the #3 priority.
With our priorities assigned, we look next at the #4 priority group (the hydrogen) and see that it is pointed back away from us, into the plane of the page – thus step 4a from the procedure above applies. Then, we trace a circle defined by the #1, #2, and #3 priority groups, in increasing order. The circle is clockwise, which by step 4a tells us that this carbon has the ‘R’ configuration, and that this molecule is (R)-glyceraldehyde.
Its enantiomer, by definition, would be (S)-glyceraldehyde.
As another example, let’s look at one of the enantiomers of lactic acid and determine the configuration of the chiral center.
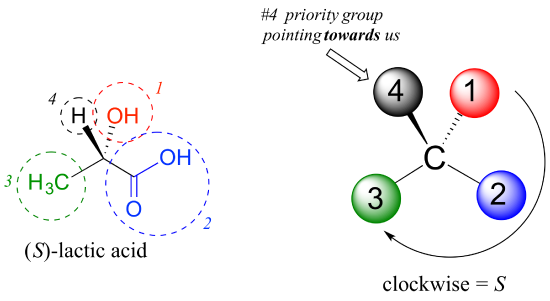
An Interactive model of (S)-alanine is available by following this link.
Clearly, H is the #4 substituent and OH is #1. Owing to its three bonds to oxygen, the carbon on the acid group takes priority #2, and the methyl group takes #3. The #4 group, hydrogen, happens to be drawn pointing toward us (out of the plane of the page) in this figure, so we use step 4b: The circle traced from #1 to #2 to #3 is clockwise, which means that the chiral center has the S configuration.
The drug thalidomide is an interesting – but tragic – case study in the importance of stereochemistry in drug design. First manufactured by a German drug company and prescribed widely in Europe and Australia in the late 1950’s as a sedative and remedy for morning sickness in pregnant women, thalidomide was soon implicated as the cause of devastating birth defects in babies born to women who had taken it. Thalidomide contains a chiral center, and thus exists in two enantiomeric forms. It was marketed as a racemic mixture: in other words, a 50:50 mixture of both enantiomers.
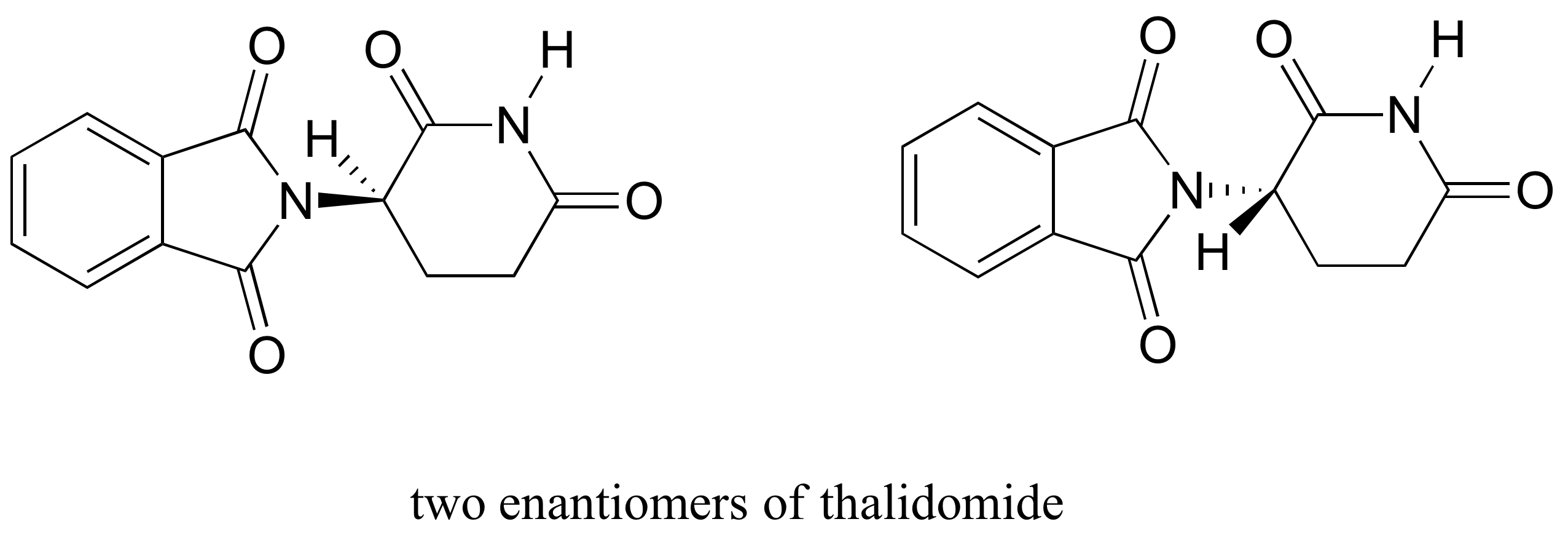
Although scientists are still not entirely sure how thalidomide works, experimental evidence suggests that it was actually the R enantiomer that had the desired medical effects, while the S enantiomer caused the birth defects. Even with this knowledge, however, pure (R)-thalidomide is not safe, because enzymes in the body rapidly convert between the two enantiomers..

As a historical note, thalidomide was never approved for use in the United States. This was thanks in large part to the efforts of Dr. Frances Kelsey, a Food and Drug pharmacologist and physician, who, at peril to her career and resisting pressure, blocked its approval due to her concerns about the lack of adequate safety studies, particularly with regard to the drug’s ability to enter the bloodstream of a developing fetus. Unfortunately, though, at that time clinical trials for new drugs involved widespread and unregulated distribution to doctors and their patients across the country, so families in the U.S. were not spared from the damage caused.
Kelsey’s work and public awareness of it led directly to a strengthened process for drug approvals. This included passage of the Kefauver Harris Amendment, which required companies demonstrate the efficacy of new drugs, report adverse reactions to the FDA, and request consent from patients participating in clinical studies before approval and marketing.
Thalidomide is now back on the market, with strict safety measures enforced, for the treatment of multiple myeloma, graft vs. host disease, and some conditions related to leprosy. Disturbingly, control of the drug has been less than perfect in some parts of the world, leading to continuing problems, for instance in Brazil.
Exercise 6.2.1
Exercise 6.2.2
Exercise 6.2.3
Determine the stereochemical configurations of the chiral centers in the biomolecules shown below.
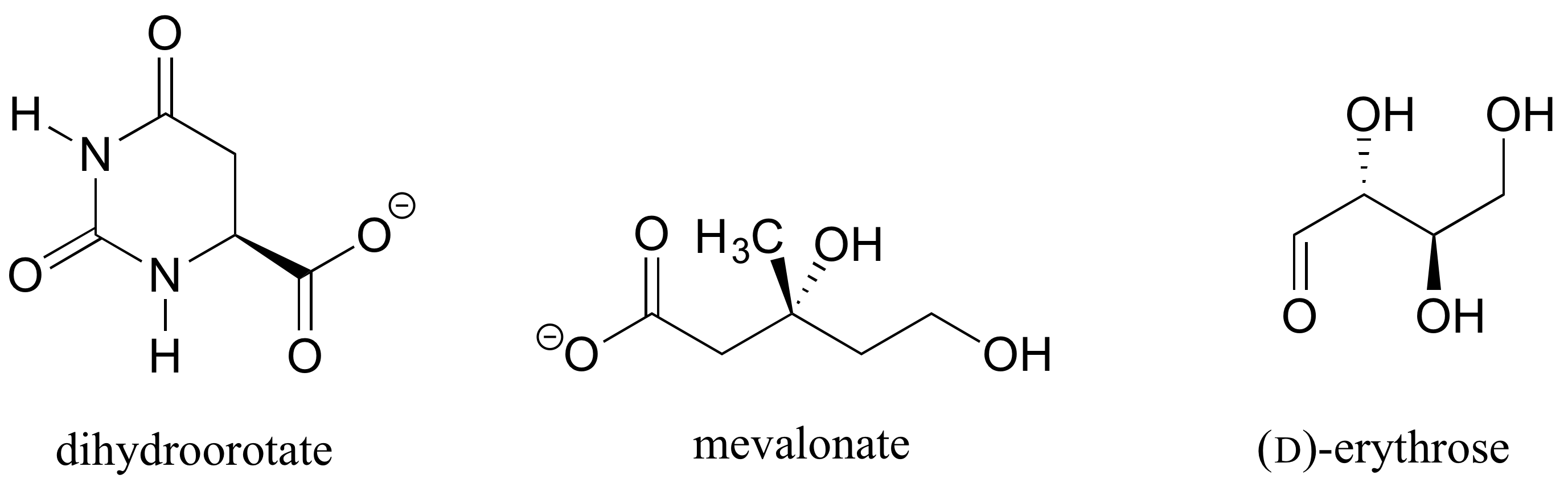
Exercise 6.2.4
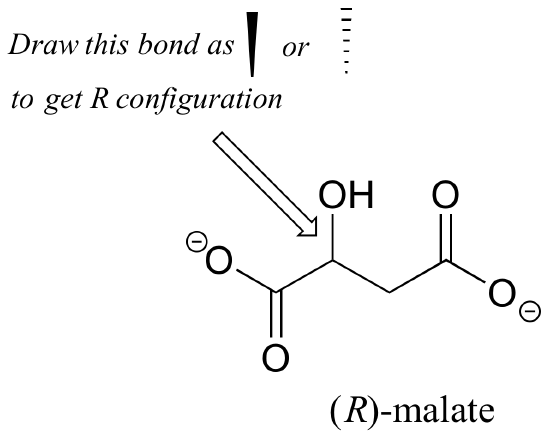
Should the (R) enantiomer of malate have a solid or dashed wedge for the C-O bond in the figure below?