
Carbohydrates are commonly described as sugars, or saccharides, from the Greek word for sugar. The simplest carbohydrates are called monosaccharides. An example is glucose. Monosaccharides can be joined to make larger molecules. Disaccharides contain two monosaccharides. Sucrose is a disaccharide, containing both fructose and glucose. Mono and disaccharides are sometimes referred to as simple sugars. Polysaccharides are chains of many sugar subunits. Examples include glycogen and cellulose, both of which are polymers of glucose (configured differently).
Carbohydrates are literally “hydrates of carbon.” This name derives from the generalized formula of simple monosaccharides, which can be written in the form of Cx(H2O)x, where x is a digit typically between 3 and 8. Not all sugars have this formula, however. Deoxyribose, the sugar found in every nucleotide in a DNA molecule lacks one oxygen and thus has the formula C5H10O4.
Carbohydrates are important in cells as energy sources (especially glucose, glycogen, and amylose), as markers of cellular identity (oligosaccharides on the surface of cells of multicellular organisms), as structural components (cellulose holding up plants), and as constituents of nucleotides (ribose in RNA, deoxyribose in DNA).
The building blocks of all carbohydrates are the monosaccharides.
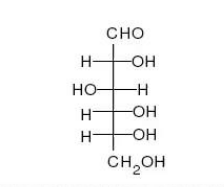
Shown below are Fischer projection formulas for a group of common monosaccharides. Fischer projection formulas are similar but not identical to organic structural formulas. Carbons in the sugar are represented with the elemental symbol C at the end of the chain, but also are represented by vertices (such as carbon 1 in D-Ribose below) and by intersecting perpendicular lines (carbons 2, 3, and 4 in D-Ribose).
The tetrahedral arrangement around the carbons in the chain of a monosaccharide are represented as flat, with 90 degree bond angles, in the Fischer projection. This is not an accurate representation of the three-dimensional molecules. To interpret these structures as 3D models, each carbon within the chain can be considered in sequence. The bonds shown vertically in the Fischer projection are oriented back, away from the viewer, while the horizontal bonds (to H and OH) emerge forward, out of the plane of view.
Fischer projections make for easy drawing and comparison of carbohydrate structure but their interpretation is prone to error.
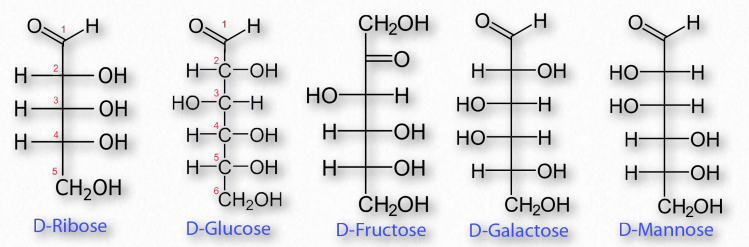
Figure 2.148 – Common sugar structures
Monosaccharides
The most common monosaccharides include glucose, fructose, galactose, ribose, and mannose. Of these sugars, all but one (fructose) exists as an aldehyde. Fructose and some other less well known sugars are ketones. Figure 2.148 shows the structure of these sugars.
By convention, the letters ‘ose’ at the end of a biochemical name flags a molecule as a sugar. Thus, there are glucose, galactose, sucrose, and many other ‘-oses’. Other descriptive nomenclature involves use of a prefix that tells how many carbons the sugar contains. For example, glucose, which contains six carbons, is described as a hexose. The following list shows the prefixes for numbers of carbons in a sugar:
•Tri- = 3
•Tetr- = 4
•Pent- = 5
•Hex- =6
•Hept- = 7
•Oct- = 8
Other prefixes identify whether the sugar contains an aldehyde group (aldo-) or a ketone (keto-) group. Prefixes may be combined. Glucose, which is a 6-carbon sugar with an aldehyde group, can be described as an aldohexose. The list that follows gives the common sugars and their descriptors. •Ribose = aldo-pentose
•Glucose = aldo-hexose
•Galactose = aldo-hexose
•Mannose = aldo-hexose
•Fructose = keto-hexose
Diastereomers
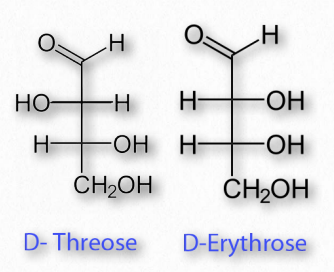
Figure 2.149 – Diastereomers
Sugars may have multiple chiral carbons and thus differ from each other in the configuration of groups around those asymmetric carbons. Two sugars having the same chemical form (aldoses, for example) and the same number of carbons, but that differ only in the stereochemical orientations of their carbons are referred to as diastereomers (Figure 2.149). For example, glucose, galactose, and mannose all have the formula of C6H12O6, but are chemically distinct from each other in the orientation of groups around the carbons within them.
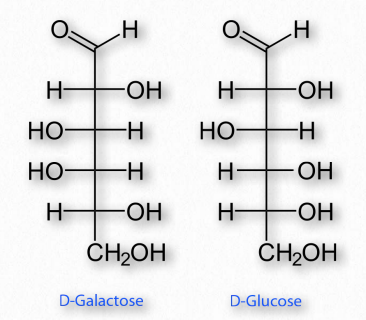
Figure 2.150 – Epimers – D-Galactose and D-Glucose differ only in the configuration of carbon #4
Enantiomers and epimers
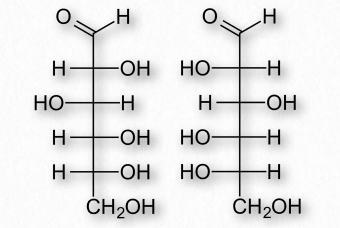
Figure 2.151 – Enantiomers – D-Glucose (left) and L-Glucose (right) are mirror
If two sugars are identical except for having one chiral carbon arranged differently (such as images glucose and galactose – Figure 2.150), they are considered epimers of one another. If two sugars are mirror images of each other, they are enantiomers (Figure 2.151). Biochemical notation uses the letters D and L to describe monosaccharide stereochemistry in a very particular way. As a result, one enantiomer will be given an L designation while the other is D. So L-glucose is the mirror image of D-glucose.
Movie 2.6 – Conversion of glucose from a straight chain form to a ring form
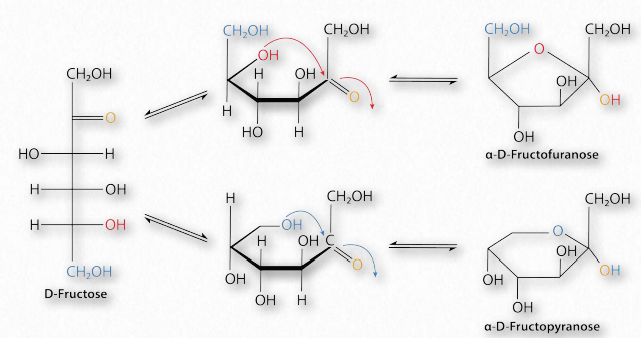
Figure 2.152 – Conversion of D-fructose between furanose (top right), linear (left), and pyranose (bottom right) forms Image by Pehr Jacobson
Sugars with five and six carbons can readily cyclize (Figure 2.152, Movie 2.6) in solution. When they do, a new asymmetric carbon is created that didn’t exist in the same sugars when they were in the straight chain form, as the carbon to oxygen double bond converts to an alcohol. This carbon has a special name – it is called the anomeric carbon and (like the other asymmetric carbons in sugars) it can have the hydroxyl in two different positions. These positions are referred to as α and β . Sugars, such as α-D-glucose and β-D-glucose that differ only in the configuration of the anomeric carbon are referred to as anomers (Figure 2.153).
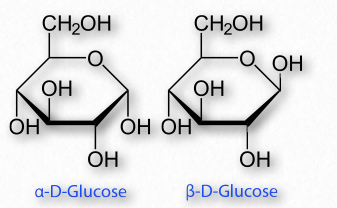
Figure 2.153 – Anomers – α-DGlucose and β-D-Glucose differ only in the configuration of the anomeric carbon #1
Sugars cyclizing to form rings with five atoms in them (see fructose in Figure 2.128) are referred to as furanoses (named for furan) and those forming rings with six atoms, such as glucose in the same figure, are called pyranoses (named for pyran). The carbonyl carbon becomes the anomeric carbon in the ring by binding to the oxygen of a hydroxyl elsewhere in the chain. α- and β- forms of a given sugar can readily “flip” between each form in solution, so long as the anomeric hydroxyl is free, because the bonding in cyclic forms is unstable, so molecules interconvert in solution. Most pentoses and hexoses can form both furanose and pyranose structures (Figure 2.152).
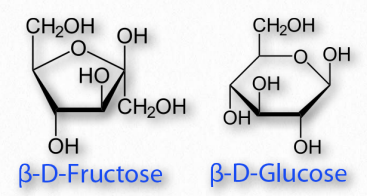
Figure 2.154 – A furanose (left) and a pyranose (right)
Linking the anomeric hydroxyl to another group will create a structure called a glycoside which will remain locked in whichever α- or β- configuration they were in when the anomeric hydroxyl was altered.
Boat/chair conformations
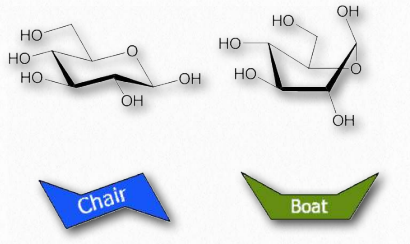
Figure 2.155 – Chair and boat forms of glucose
Orbitals of carbon prefer to be in tetrahedral conformations and this means that the bonds between carbons in a ring do not lie flat. Indeed, rings “pucker” to try to accommodate this tendency, giving rise to different 3D forms for any given sugar. Some of these forms resemble boat structures, which others resemble chairs or envelopes (Figure 2.155). The stablest (and thus most abundant) of these forms have all of the hydoxyls in the equatorial positions, resulting in less steric hindrance.
Modified monosaccharides
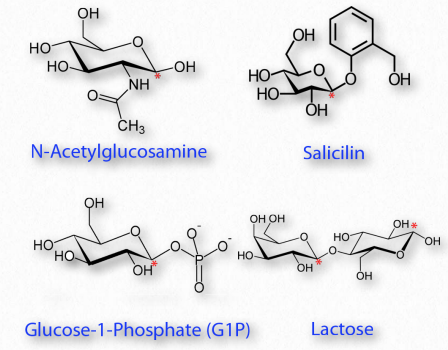
Figure 2.156 – Modified sugars. Locations of glycosidic carbon indicated with red asterisks. All are glycosides except N-acetylglucosamine
Many chemical modifications can occur on sugar residues (Figure 2.156). Common ones include oxidation, reduction, phosphorylation, and substitution of an amine or an acetylamine for a hydroxyl. The ones that affect the anomeric hydroxyl group make glycosides (Figure 2.157), whereas modifications that don’t affect the anomeric hydroxyl, (glucose-6-phosphate, for example), do not.
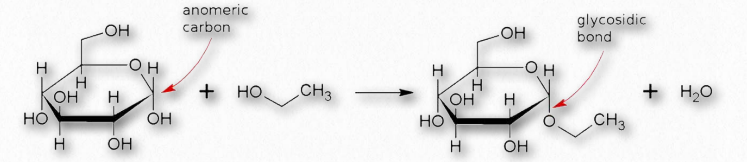
Figure 2.157 – Formation of a glycosidic bond
Oxidation/reduction

Figure 2.158 – A positive Benedict’s test starting at left and moving right Wikipedia
The last considerations for simple sugars relative to their structure are their chemical reactivity and modification. Sugars that are readily oxidized are called ‘reducing sugars’ because their oxidation causes other reacting molecules to be reduced. A test for reducing sugars is known as Benedict’s test. In it, sugars are mixed and heated with an alkaline solution containing Cu++. Reducing sugars will donate an electron to Cu++, converting it to Cu+, which will produce cuprous oxide Cu2O, as an orange precipitate (Figure 2.158). Since Cu++ solution is blue, the change of color provides an easy visual indication of a reducing sugar.
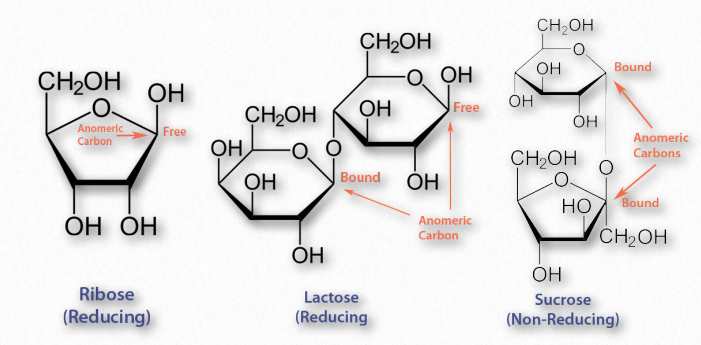
Figure 2.159 – Reducing and non-reducing sugars
The aldehyde group of aldoses is very susceptible to oxidation, whereas ketoses are less so, but can easily be oxidized if, like fructose, they contain an α-hydroxyl and can tautomerize to an aldose. Most monosaccharides are reducing sugars. This includes all of the common ones galactose, glucose, fructose, ribose, xylose, and mannose. Some disaccharides, such as lactose and maltose are reducing sugars since they have at least one anomeric carbon free, allowing that part of the sugar to linearize and yield an aldose. Sucrose, on the other hand has no anomeric carbons free – both are involved in a glycosidic linkage, so they cannot linearize and thus it is not a reducing sugar.
Oxidation and reduction of sugars can occur in cells. As we will see, phosphorylation of sugars occurs routinely during metabolism.
Glucuronic acid
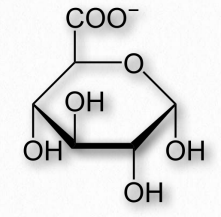
Figure 2.160 – Glucuronic acid
One oxidation product of glucose is glucuronic acid, a six carbon molecule where the CH2OH on carbon six is oxidized to a carboxylic acid (Figure 2.160). Related oxidized sugars include galacturonic acid and mannuronic acid. Glucuronic acid is commonly conjugated to other molecules in the liver/bile by UDP-glucuronyltransferase enzymes to make the molecules more water soluble for excretion, since the carboxyl group of glucoronic acid ionizes readily at physiological pH. The reactions are usually done starting with glucuronic acid linked to UDP (UDPGlucuronic Acid). In addition, glucuronic acid is made from a UDP-glucose precursor. Glucuronic acid is a common constituent of glycosaminoglycans, proteoglycans, and glycoglycerolipids. Glucuronic acid is found in heparin, dermatan sulfate, chondroitin sulfate, hyaluronic acid, and keratan sulfate. Glucuronic acid is also a precursor of ascorbic acid (Vitamin C) in organisms that synthesize this compound.
Sugar alcohols
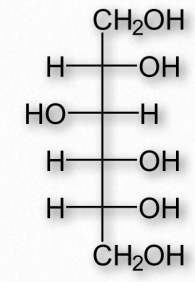
Figure 2.161 – Sorbitol (also called glucitol)
Reduction of aldoses or ketoses by hydrogenation produces the corresponding sugar alcohols. The compounds are widely used as thickeners of food or as artificial sweeteners, due to their ability to stimulate sweet receptors on the tongue. Common sugar alcohols (sugar progenitor in parentheses) include glycerol (glyceraldehyde), xylitol (xylose), sorbitol (Figure 2.161 – from glucose), galactitol (galactose), arabitol (arabinose), and ribitol (ribose). Most of these compounds have a sweetness of between 0.4 and 1.0 times as sweet as sucrose, but provide considerably fewer calories per weight. Xylitol is the sweetest of them with a sweetness equal to that of sucrose.
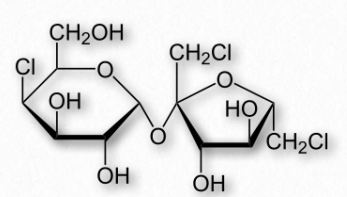
Figure 2.162 – Structure of sucralose
Sugar alcohols are used sometimes to mask the aftertaste of other artificial sweeteners. Many of them also produce a cooling sensation upon dissolving, due to that being an endothermic process for them, resulting in a pleasant mouth sensation. Last, they are poorly absorbed by intestines, and so have a low glycemic index.
Artificial sweeteners
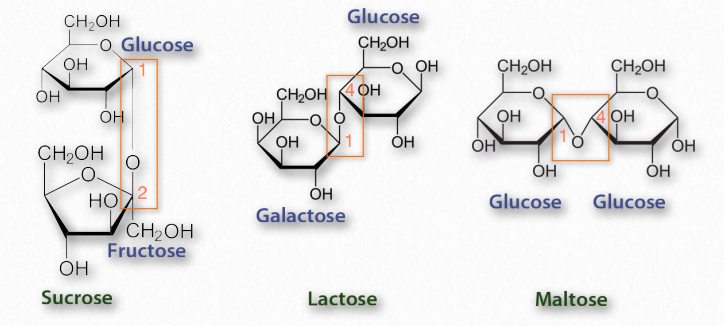
Figure 2.163 – Common disaccharides – glycosidic bonds in rectangles
Artificial sweeteners are compounds that stimulate taste receptors for sweetness, but are metabolized for energy inefficiently at best. Such compounds frequently are many times sweeter than table sugar (sucrose) on a weight/weight basis and are referred to as “intensely sweet.” Most of the artificial sweeteners are not carbohydrates, but rather are able to stimulate the same sweet receptors that sugar does. Seven such compounds are approved for use in the U.S. – stevia, aspartame, sucralose, neotame, acesulfame potassium, saccharin, 1 1 1 2 4 4 and advantame. The sugar alcohol known as sorbitol is also sometimes used as an artificial sweetener.
Disaccharides
Disaccharides (Figure 2.163) are made up of two monosaccharides. The most common ones include sucrose (glucose and fructose), lactose (galactose and glucose), and maltose (glucose and glucose). All of the common disaccharides contain at least one glycosidic bond. We name the disaccharides according to which carbons are linked to each other and the how the anomeric carbon of the glycosidic bond is configured. Lactose, for example, is described as β-Dgalactopyranosyl-(1→4)-D-glucose, or more succinctly as having an α-1,4 glycosidic bond.
Oligosaccharides
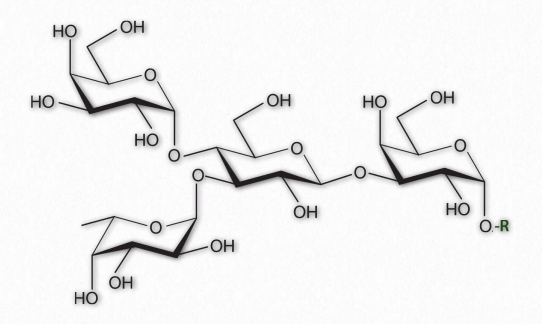
Figure 2.164 – A Branched oligosaccharide attached to an RGroup
As their name implies, oligosaccharides (Figure 2.164) are comprised of a few (typically 3 to 9) sugar residues. These often, but not always contain modified sugars. Unlike all of the other saccharides, oligosaccharides are not typically found unattached to other cellular structures. Instead, oligosaccharides are found bound, for example, to sphingolipids (making cerebrosides or gangliosides) or proteins (making glycoproteins).
Oligosaccharides in membrane glycoproteins play important roles in cellular identity/ recognition. The patterns of oligosaccharides displayed on the extracellular face of the plasma membrane acts as a sort of barcode that identifies specific cell types. The immune system recognizes these identity tags in the body. “Foreign” oligosaccharide structures trigger the immune system to attack them. While this provides a very good defense against invading cells of an organism, it also can pose significant problems when organs are transplanted from one individual into another, with rejection of donated organs, in some cases.
Organelle targeting
The oligosaccharides that are attached to proteins may also determine their cellular destinations. Improper glycosylation or errors in subsequent sugar modification patterns can result in the failure of proteins to reach the correct cellular compartment.
For example, inclusion cell disease (also called I-cell disease) arises from a defective phosphotransferase in the Golgi apparatus. This enzyme normally catalyzes the addition of a phosphate to a mannose sugar attached to a protein destined for the lysosome. In the absence of a functioning enzyme, the unphosphorylated glycoprotein never makes it to the lysosome and is instead exported out of the cell where it accumulates in the blood and is excreted in the urine. Individuals with Icell disease suffer developmental delays, abnormal skeletal development, and restricted joint movement.
Glycosylation
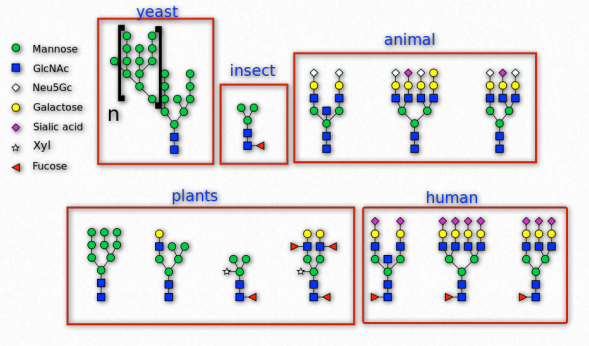
Figure 2.165 – N-linked glycosylation in various organisms Wikipedia
Sugars are commonly attached to proteins in a process called glycosylation. Typically the attachment is to a hydroxyl or other functional group. The majority of proteins synthesized in the endoplasmic reticulum are glycosylated.
N-glycans on cell surfaces play roles in the immune system. The immunoglobulin types (IgG, IgA, IgE, IgD, and IgM) have distinct glycosylation patterns that confer unique functions by affecting their affinities for immune receptors. Glycans also are important in self/non-self identity is tissue rejection and autoimmune diseases.
Glycoproteins
Figure 2.170 – Erythropoietin Wikipedia
Glycoproteins are a very diverse collection of saccharide-containing proteins with many functions. Attachment of the saccharide to the protein is known as glycosylation. Secreted extracellular proteins and membrane proteins with exposed extracellular regions are often glycosylated. Saccharides attached to these may be short (oligosaccharides) or very large (polysaccharides). Glycoproteins play important roles in the immune system in antibodies and as components of the major histocompatibility complex (MHC). They are important for interactions between sperms and eggs, in connective tissues and are abundant in egg whites and blood plasma. Two glycoproteins (gp41 and gp120) are part of the HIV viral coat and are important in the infection process. Some hormones, such as erythropoietin, human chorionic gonadotropin, follicle-stimulating hormone and luteinizing hormone are also glycoproteins.
Glycation
Glycation is a chemical process (nonenzymatic) that occurs when a protein or lipid covalently binds to a sugar, such as glucose or fructose. Glycation differs from glycosylation in that the latter process is controlled by enzymes and results in specific attachment of specific sugars to biomolecules. Glycation, by contrast, is driven by two properties of monosaccharides 1) their chemistry and 2) their concentration. Glycations may be endogenous (occurring in an organism) or exogenous (occurring external to an organism).
Exogenous glycation arises most commonly as a result of cooking of food and this results in attachment of sugars to lipids and/or proteins to form advanced glycation endproducts (AGEs). At temperatures above 120°C, AGE production occurs readily and contributes to the taste and the appearance of the food we eat.
Cooking
Browning of food, for example, is a product of glycation and is enhanced as the sugar content of a food increases. Browning of french fries is often enhanced, for example, by adding sugar to them. The formation of a crust of bread or the toasting of bread are other examples. These glycations are products of the Maillard reaction in which a reactive sugar carbonyl group combines with a nucleophilic amine of an amino acid. The process is favored in an alkaline environment, when amines are less protonated. The formation of the harder shell of a pretzel, for example, results from addition of lye to the exterior. At higher temperatures, though, a carcinogen known as acrylamide can be formed by reactions involving asparagine.
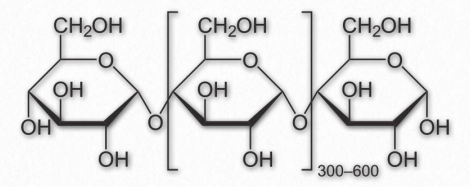
Figure 2.171 – Repeating unit of amylose
Endogenous glycation, on the other hand, arises with a frequency that is proportional to the concentration of free sugar in the body. These occur most frequently with fructose, galactose, and glucose in that decreasing order and are detected in the bloodstream. Both proteins and lipids can be glycated and the accumulation of endogenous advanced glycation endproducts (AGEs) is associated with Type 2 diabetes, as well as in increases in cardiovascular disease (damage to endothelium, cartilage, and fibrinogen), peripheral neuropathy (attack of myelin sheath), and deafness (loss of myelin sheath).
The formation of AGEs increases oxidative stress, but is also thought to be exacerbated by it. Increased oxidative stress, in turn causes additional harm. Damage to collagen in blood cells causes them to stiffen and weaken and is a factor in hardening of the arteries and formation of aneurysms, respectively. One indicator of diabetes is increased glycation of hemoglobin in red blood cells, since circulating sugar concentration are high in the blood of diabetics. Hemoglobin glycation is measured in testing for blood glucose control in diabetic patients.
| Homopolymer | Monomeric Unit |
| Glycogen | Glucose |
| Cellulose | Glucose |
| Amylose | Glucose |
| Callose | Glucose |
| Chitin | N-acetylglucosamine |
| Xylan | Xylose |
| Mannan | Mannose |
| Chrysolaminarin | Glucose |
Polysaccharides
Long polymers of sugar residues are called polysaccharides and can be up to many thousands of units long. Polysaccharides are found free (not attached to other molecules) or bound to other cellular structures such as proteins. Some polysaccharides are homopolymers (contain only one kind of sugar). Others are heteropolymers (glycosaminoglycans, hemicellulose). Polysaccharides function in energy storage (nutritional polysaccharides, such as glycogen, amylose, amylopectin, e.g.), structure enhancement (chitin, cellulose, e.g.), and lubrication (hyaluronic acid, e.g.). These individual categories of polysaccharides are discussed below.
Nutritional polysaccharides
This group of polysaccharides is used exclusively for storage of sugar residues. They are easily easily broken down by the organism making them, allowing for rapid release of sugar to meet rapidly changing energy needs.
Amylose
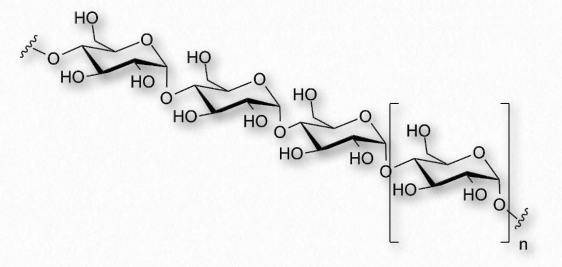
Figure 2.172 – Another view of amylose
Amylose has the simplest structure of any of the nutritional polysaccharides, being made up solely of glucose polymers linked only by α-1,4 bonds (Figure 2.171 & 2.172). (Note that the term ‘starch’ is actually a mixture of amylose and amylopectin). Amylose is insoluble in water and is harder to digest than amylopectin (see below). The complexing of amylopectin with amylose facilitates its water Figure 2.172 – Another view of amylose solubility and its digestion. Amylose is produced in plants for energy storage and since plants don’t have rapidly changing demands for glucose (no muscular contraction, for example), its compact structure and slow breakdown characteristics are consistent with plants’ needs.
Amylopectin and glycogen
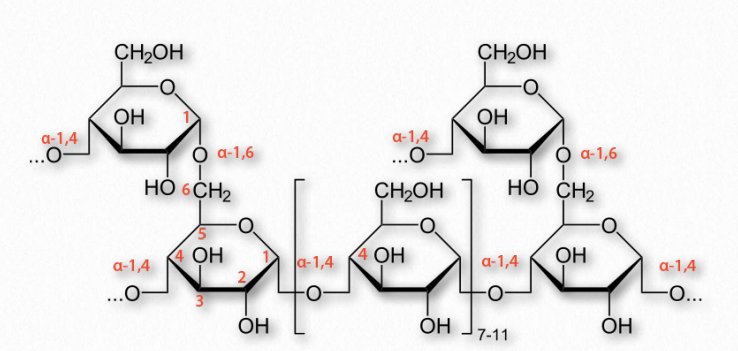
Figure 2.173 – Structure of glycogen
More complicated homopolymers of glucose are possessed by amylopectin in plants and glycogen (Figure 2.173) in animals. Both compounds contain long glucose chains with α-1,4 bonds like amylose, but unlike amylose, these long chains have branches of α-1,6 bonds. Amylopectin is the less-branched of the two, having such bonds about every 25-30 residues, whereas glycogen has branches about every 8-12 residues.
Branching plays important roles in increasing water solubility and in providing more “ends” to the polymer. In animals, glycogen is broken down starting at the ends, so more ends means more glucose can be released quickly. Again, plants, which have a lower need for quick release of glucose than animals get by with less branching and fewer ends.
Structural polysaccharides
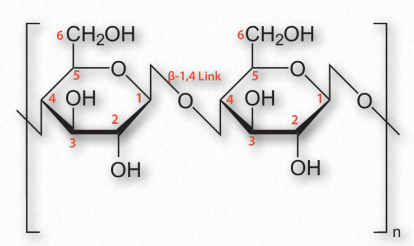
Figure 2.174 – Cellulose with β-1,4 links between glucose sugars
An additional function of polysaccharides in cells relates to structure. Cellulose, which is a polymer of glucose with exclusive β-1,4 linkages between the units (Figure 2.174) is an important structural component of plants and fungi cells. Notably, most non-ruminant animals are unable to digest this polymer, as they lack the enzyme known as cellulase.
Ruminants, such as cattle, however, contain in their rumen a bacterium that possesses this enzyme and allows them to obtain glucose energy from plants. Another group of polysaccharides found in plant cell walls is the hemicelluloses. This class of molecules encompasses several branched heteropolymers of (mostly) D-pentose sugars along with a few hexoses and L-sugars as well. Hemicelluloses are shorter than cellulose (500-3000 sugars versus 7000-15,000 sugars).
Monomer sugars of polysaccharides besides glucose include xylose, mannose, galactose, rhamnose, and arabinose. Xylose is usually present in the greatest amount (Figure 2.175).
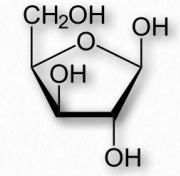
Figure 2.175 Xylose
Chitin
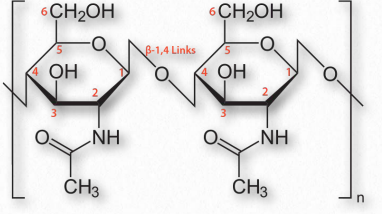
Figure 2.176 – Chitin with β-1,4 links between N-acetylglucosamine sugars
Chitin (Figure 2.176) is another structural polysaccharide, being comprised of N-acetylglucosamine units joined by β-1,4 linkages. It is a primary component of the cell walls of fungi and is also prominent in the exoskeletons of arthropods and insects, as well as the beaks and internal shells of cephalopods (Figure 2.177). Chitin’s structure was solved by Albert Hofmann in 1929. It is like cellulose except for the acetylamine group replacing the hydroxyl on position 2. This change allows hydrogen bonding to occur between adjacent polymers, thus providing greater strength.
Pectins
Another group of structural polysaccharides is the pectins (Figure 2.178). These com pounds are present in most primary plant cell walls and are abundant in non-woody parts of terrestrial plants. They are rich in galacturonic acid (α-1,4 links with no branches – Figure 2.179) and are used commercially as a gelling agent in jams/jellies, as well as a stabilizer in fruit juices and milk drinks. Pectin consumption may result in reduced blood cholesterol levels due to its tendency to 1) bind cholesterol and 2) to increase viscosity in the intestinal tract, thus reducing absorption of cholesterol from food. Pectins also trap carbohydrates in the digestive system and reduce their rate of absorption.
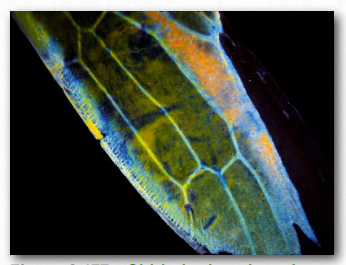
Figure 2.177 – Chitin in the wing of a sap beetle Wikipedia

Figure 2.178 – A powdered form of pectin
Lectins
Lectins are not carbohydrates, but proteins that specifically bind to carbohydrate molecules found in animals and plants (where they are known as phytohemagglutinins) and are each highly specific for certain sugars. They function in cellular and molecular recognition, as well as cell adhesion. One lectin recognizes hydrolytic enzymes containing mannose-6-phosphate and targets them to be delivered to lysosomes. In the innate immune system, a mannose binding lectin helps defend against invading microbes. Other lectins have roles in inflammation and autoimmune disorders.
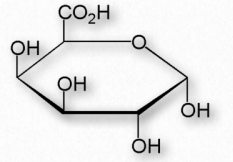
Figure 2.179 – α-DGalacturonic acid – An important component of pectin polymers
Some viruses and bacteria use lectins to recognize and bind specific carbohydrate residues on the surface of target cells. Flu virus, for example, carries a lectin known as hemagglutinin (Figure 2.180) that binds to sialic acid and is essential for entrance of the virus into the target cell. After binding, the viral particle enters by endocytosis after the hemagglutinin has been cleaved by a protease.
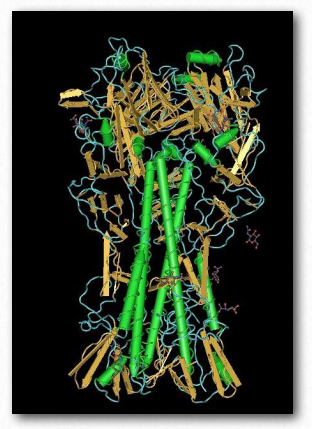
Figure 2.180 – Hemagglutinin
After replication of the virus inside of the cell, hemagglutinin and and a viral enzyme known as neuraminidase cluster in the cell membrane. Viral RNA and associated viral proteins cluster near this membrane site and new viruses bud off in a portion of the cell’s membrane after the hemagglutinin-sialic acid link to the infected cell is released by the neuraminidase cutting the bond between the sialic acid and the rest of the cell surface carbohydrate. Drugs, such as tamiflu, that interfere with neuraminidase work by preventing release of the viral particle. Unreleased particles will tend to aggregate and not function.
Some viral glycoproteins from hepatitis C virus may attach to lectins on the surface of liver cells in their infectious cycle. The bacterium Helicobacter pylori uses a cell surface lectin to bind oligosaccharides on epithelial cells lining the stomach. One lectin known as ricin is a very powerful toxin. It is produced in the endosperm of seeds of the castor oil plant and is of concern as a bioterrorism weapon as a result of its acute toxicity when inhaled or ingested.
Lectins were discovered originally in plants and have been most studied in legumes, but lectins are now known to be widely dispersed in nature. In the immune system, a mannan binding lectin (MBL) helps mediate the first defenses against microorganisms. Other immune system lectins are thought to modulate inflammatory processes and probably play a role in self/non-self recognition that is at the root of rejection of transplanted organs.
Heparin
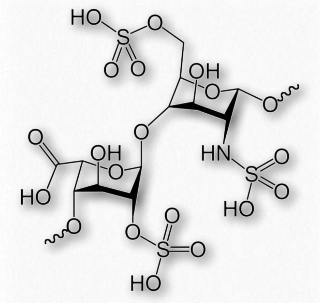
Figure 2.186 – Repeating sulfated disaccharide in heparin
Heparin (Figures 2.186 & 2.187) is a modified polysaccharide whose biological function is unclear, but whose ability to prevent clotting of blood is used for medical purposes. Heparin does not dissolve blood clots. Rather, it acts to prevent conversion of fibrinogen to fibrin.
Whether or not heparin is actually used by the body for its anticoagulation property is uncertain. It is stored in the secretory granules of mast cells and released at the point of injury and it has been proposed it is a protection against bacteria and other foreign materials.
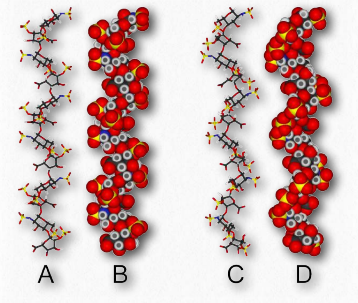
Figure 2.187 – Two structures for heparin
Heparin has abundant sulfates and is, in fact, the molecule with the highest negative charge density known. Its size varies from 3 kDa to 30 kDa, with an average of about 15 kDa. The repeating disaccharide of 2-Osulfated iduronic acid and 6-O-sulfated, N- sulfated glucosamine, occupies about 85% of the molecule. Copper salts of heparin help stimulate the synthesis of blood vessels (angiogenic).
Hyaluronic acid
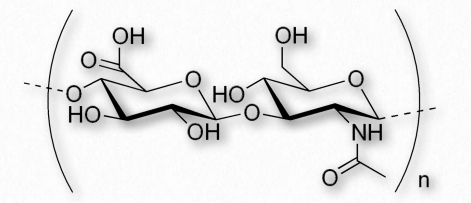
Figure 2.188 – Repeating disaccharide of hyaluronic acid
Hyaluronic acid (also known as hyaluronan or hyaluronate) is a glycosaminoglycan found in connective, epithelial, and nerve tissues. It is an unusual glycosaminoglycan (Figure 2.188), lacking sulfate, is made by hyaluronan synthases on the inner face of the plasma membrane and has a molecular weight in the millions. An average adult body contains about 15 grams of HA, one third of which is replaced every day. The repeating unit in hyaluronic acid is a disaccharide structure of D-glucuronic acid joined to D-N-acetylglucosamine. The compound, which can have upwards of 25,000 units of the disaccharide, is delivered directly into the extracellular matrix by enzymes from its plasma membrane site of synthesis.It is an important component of the extracellular matrix, where it assists in cell proliferation and migration. The polymer provides an open hydrated matrix to facilitate general cell migration whereas directed cell migration occurs via the interaction between hyaluronic acid and specific cell surface receptors. HA interaction with the receptor RHAMM (Receptor for Hyaluronan Mediated Motility) has been shown to be involved in wound repair as well as tumor progression.
Synovial Fluid
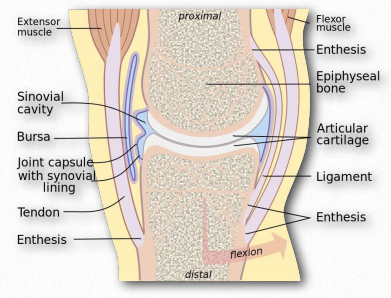
Figure 2.189 – Synovial fluid in joint lubrication Wikipedia
The function of hyaluronic acid has traditionally been described as providing lubrication in synovial fluid (the lubricating material in animal joints – Figure 2.189). Along with the proteoglycan called lubricin, hyaluronic acid turns water into lubricating material. Hyaluronic acid is present as a coat around each cell of articular cartilage and forms complexes with proteoglycans that absorb water, giving resilience (resistance to compression) to cartilage. Aging causes a decrease in size of hyaluronans, but an increase in concentration.
Function in skin
Hyaluronic acid is a major component of skin and has functions in tissue repair. With exposure to excess UVB radiation, cells in the dermis produce less hyaluronan and increase its degradation.
For some cancers the plasma level of hyaluronic acid correlates with malignancy. Hyaluronic acid levels have been used as a marker for prostate and breast cancer and to follow disease progression. The compound can to used to induce healing after cataract surgery. Hyaluronic acid is also abundant in the granulation tissue matrix that replaces a fibrin clot during the healing of wounds. In wound healing, it is thought that large polymers of hyaluronic acid appear early and they physically make room for white blood cells to mediate an immune response.
Breakdown
Breakdown of hyaluronic acid is catalyzed by enzymes known as hyaluronidases. Humans have seven types of such enzymes, some of which act as tumor suppressors. Smaller hyaluronan fragments can induce inflammatory response in macrophages and dendritic cells after tissue damage. They can also perform proangiogenic functions.

