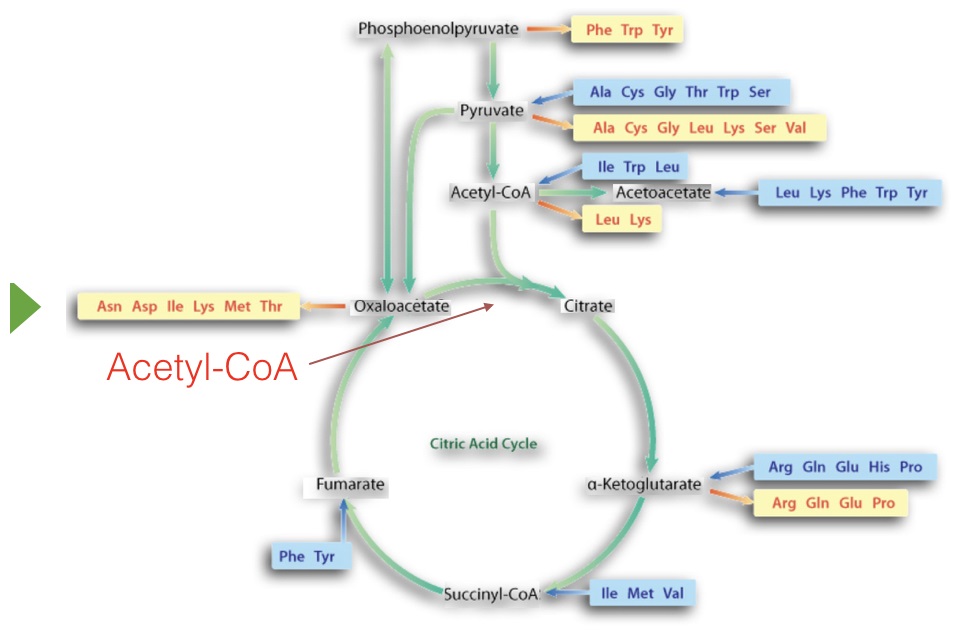
Figure 6.63 – Amino acid metabolism and the citric acid cycle. Amino acids boxed in yellow are made from the indicated intermediate. Amino acids in blue are made into the intermediate in catabolism. Image by Aleia Kim
Acetyl-CoA
The molecule “feeding” the citric acid cycle by bringing in new carbons is acetyl-CoA. It can be obtained from pyruvate (from the catabolism of carbohydrates via a pathway called glycolysis), from the fatty acid β-oxidation pathway, from ketone bodies, and from amino acid metabolism. It is worth noting that acetyl-CoA has very different fates, depending on the cell’s energy status/needs. In other words, the pathway has branches out as well as multiple materials that can feed in to it. The description below describes oxidation (catabolism) in citric acid cycle.
Before discussing the citric acid cycle, it is important to first describe one important enzyme complex that is a major source of acetyl-CoA for the cycle.
Figure 6.64 – E1 Subunit of Pyruvate Dehydrogenase. Wikipedia
Pyruvate decarboxylation
The pyruvate dehydrogenase enzyme is a complex of multiple copies of three subunits that catalyze the decarboxylation (removal of CO2 from) of pyruvate to form acetyl-CoA. The reaction mechanism requires use of five coenzymes, including thiamin pyrophosphate which is derived from the B vitamin named thiamin. Pyruvate dehydrogenase is an enormous complex in mammals with a size five times greater than ribosomes.
Puruvate dehydrogenase produces the reduced nucleotide NADH as well as Acetyl-CoA. As with other sources of NADH or FADH2, the reduced nucleotide can be used as a source of electrons for the Electron Transport Chain, leading to the formation of ATP via oxidative phosphorylation.
Pyruvate dehydrogenase regulation
Pyruvate deyhdrogenase is regulated both allosterically and by covalent modification – phosphorylation / dephosphorylation. Regulation of pyruvate dehydrogenase, whether by allosteric or covalent mechanisms has the same strategy. Indicators of high energy availability in a cell shut down the enzyme, whereas indicators of low energy stimulate it.
For allosteric regulation, the high energy indicators affecting the enzyme are ATP, acetyl-CoA, NADH, and fatty acids, which inhibit it. AMP, Coenzyme A, NAD+, and calcium, on the other hand, stimulate it (Figure 6.67).
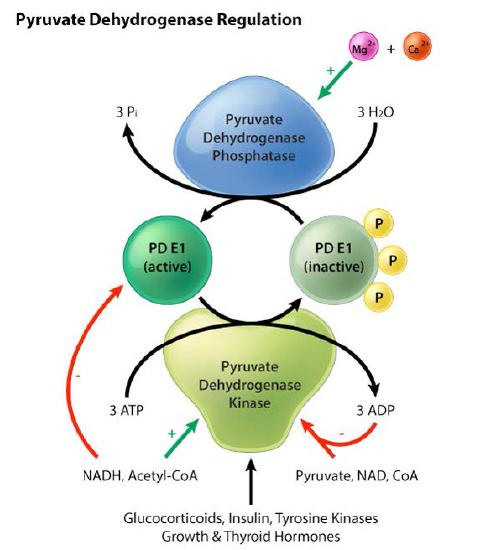
Figure 6.67 – Regulation scheme for pyruvate dehydrogenase (PD). Image by Aleia Kim
Covalent modification regulation of pyruvate dehydrogenase is a bit more complicated. It occurs as a result of phosphorylation by pyruvate dehydrogenase kinase (PDK – Figure 6.67) or dephosphorylation by pyruvate dehydrogenase phosphatase (PDP). PDK is allosterically activated in the mitochondrial matrix when NADH and acetyl-CoA concentrations rise. While the mechanism is a bit complex, the activation or deactivation of the complex by phosphorylation is aligned with energy needs of the cell: when energy is very available, the complex is deactivated, and when more energy is needed, the complex is activated.
Other signals also affect the activity of the complex. For instance the hormone insulin also also activates pyruvate kinase and the glycolysis pathway, spurring cellular use of internalized glucose.
Figure 6.68 – Pyruvate dehydrogenase complex with three phosphorylation sites in red marked by arrows.Wikipedia
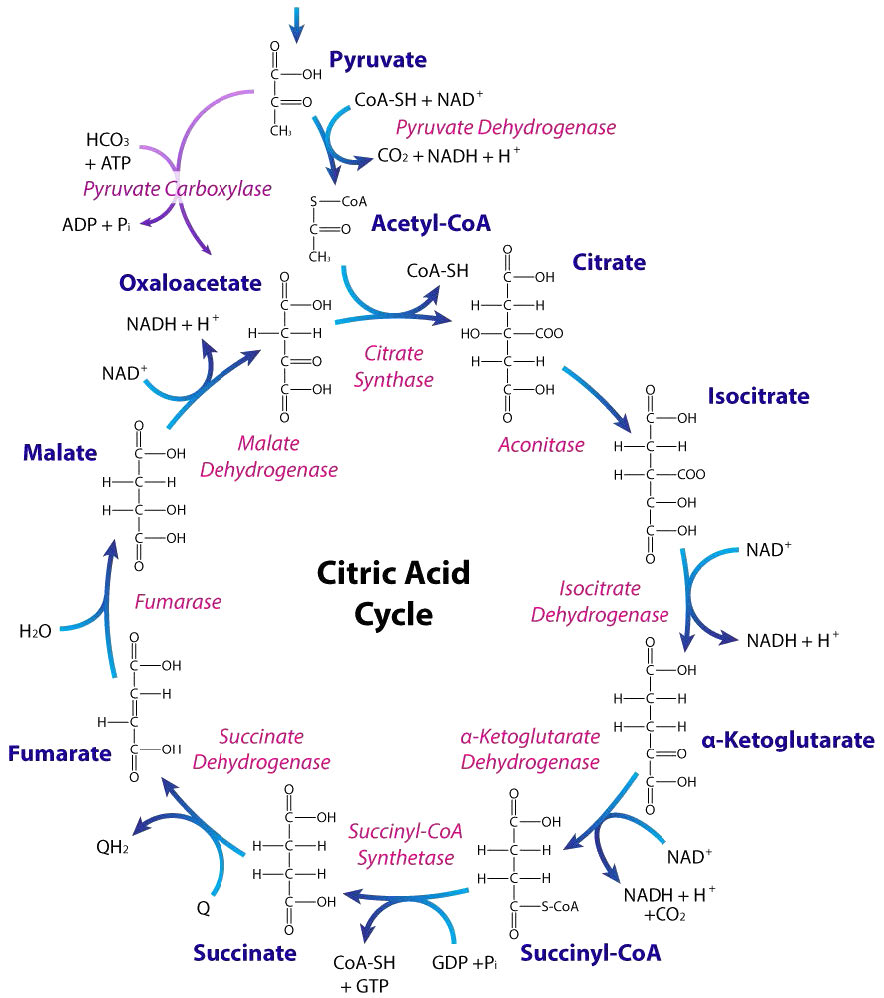
Figure 6.69 – The citric acid cycle. Image by Aleia Kim
Citric acid cycle reactions
The usual starting point point for consideration of the Citric Acid Cycle itself is the addition of acetyl-CoA to oxaloacetate (OAA) to form citrate.
Acetyl-CoA for the pathway can come from a variety of sources. The reaction joining it to OAA is catalyzed by citrate synthase and is quite energetically downhill. This, in turn, helps to “pull” the malate dehydrogenase reaction preceding it in the cycle.
In the next reaction, citrate is isomerized to isocitrate by action of the enzyme called aconitase.
Isocitrate is a branch point in plants and bacteria for the glyoxylate cycle.
Oxidative decarboxylation of isocitrate by isocitrate dehydrogenase produces the first NADH and yields α-ketoglutarate.
This five carbon intermediate is a branch point for synthesis of the amino acid glutamate. In addition, glutamate can also be made easily into this intermediate in the reverse reaction. Decarboxylation of α-ketoglutarate produces succinyl-CoA and is catalyzed by α-ketoglutarate dehydrogenase.
The enzyme α-ketoglutarate dehydrogenase is structurally very similar to pyruvate dehydrogenase and employs the same five coenzymes – NAD+, FAD, CoA-SH, thiamine pyrophosphate, and lipoamide.
These coenzymes include derivatives of the vitamins Niacin, Riboflavin, and Thiamin.
Note that at this point the cycle has produced 2 NADH molecules (reducing NAD+) during oxidation-reduction reactions. Two carbons have been ‘lost’ from the cycle as carbon dioxide, leaving four carbons from the 6-carbon citrate.
The reduced NADH can feed the Electron Transport Chain, contributing to the formation of ATP via oxidative phosphorylation.
Regeneration of oxaloacetate
The remainder of the citric acid cycle involves conversion of the four carbon succinyl-CoA into oxaloacetate. Succinyl-CoA is a branch point for the synthesis of heme. Succinyl-CoA is converted to succinate in a reaction catalyzed by succinyl-CoA synthetase (named for the reverse reaction) and a GTP is produced, as well – the only substrate level phosphorylation in the cycle.
The energy for the synthesis of the GTP comes from energy released during hydrolysis of the thioester bond between succinate and the CoA-SH.
Succinate Oxidation
Oxidation of succinate occurs in the next step, catalyzed by succinate dehydrogenase. This interesting enzyme both catalyzes this reaction and participates in the electron transport system, funneling electrons from the FADH2 it gains in the reaction to coenzyme Q. The product of the reaction, fumarate, gains a water across its trans double bond in the next reaction, catalyzed by fumarase to form malate.
Fumarate is also a byproduct of nucleotide metabolism and of the urea cycle. Malate is important also for transporting electrons across membranes in the malate-aspartate shuttle and in ferrying carbon dioxide from mesophyll cells to bundle sheath cells in C4 plants.
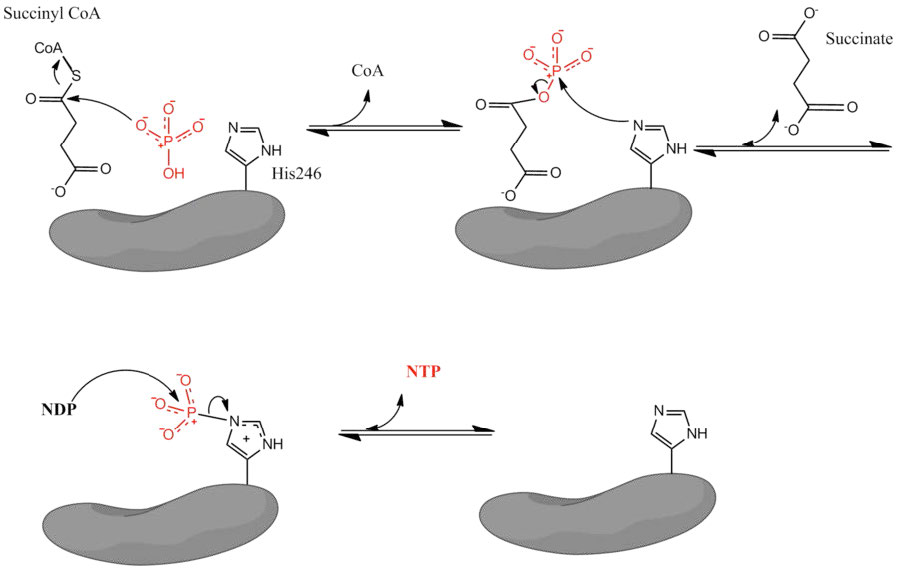
Figure 6.70 – Succinyl-CoA synthetase mechanism
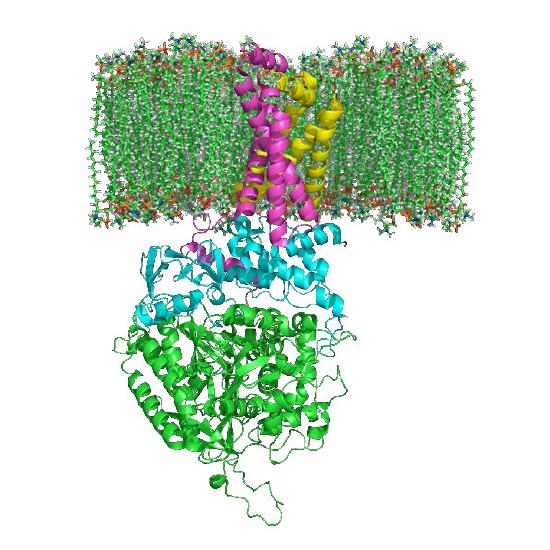
Figure 6.71 – Succinate dehydrogenase embedded in the mitochondrial inner membrane (top). Wikipedia
Rare oxidation
Conversion of malate to oxaloacetate by malate dehydrogenase is a rare biological oxidation that is energetically unfavorable on its own.
The reaction is ‘pulled’ by the energetically favorable conversion of oxaloacetate to citrate in the citrate synthase reaction described above. Oxaloacetate intersects other important pathways, including amino acid metabolism (readily converted to aspartic acid), transamination (nitrogen movement) and gluconeogenesis.
Regulation of the citric acid cycle
Allosteric regulation of the citric acid cycle occurs as substrates and products of the pathway, or molecules involved in energy transfer, activate or inhibit enzymes in ways that allow for appropriate responses by the cell to energy states. Substrates/products that regulate or affect the pathway include acetyl-CoA and succinyl-CoA .
High energy molecular indicators, such as ATP and NADH will tend to inhibit the cycle and low energy indicators (NAD+, AMP, and ADP) will tend to activate the cycle.
Regulated enzymes
Regulated enzymes in the cycle include citrate synthase (inhibited by NADH, ATP, and succinyl-CoA), isocitrate dehydrogenase (inhibited by ATP, activated by ADP and NAD+), and α-ketoglutarate dehydrogenase (inhibited by NADH and succinyl-CoA and activated by AMP).
As tends to occur in metabolism, regulation occurs on enzymes that catalyze reactions with large energy drops and which are at key branch points, shutting off entire paths that do not need to be active.
The citric acid cycle is a major catabolic pathway producing a considerable amount of energy for cells. Organisms that have the biochemical machinery to perform oxidative phosphorylation use the cycle to extract far more energy from their food than those that do not have this ability.
Since the electron transport chain uses molecular oxygen (O2) as its final electron acceptor, the citrate cycle is only beneficial as an energy source for organisms that can metabolize aerobically (with oxygen). Anaerobic organisms do not have mitochondria, do not carry out these reactions and are at a disadvantage energetically as a result. However they often can exist in ecological niches where oxygen is scarce or non-existent, and where they do not need to compete with aerobic life.
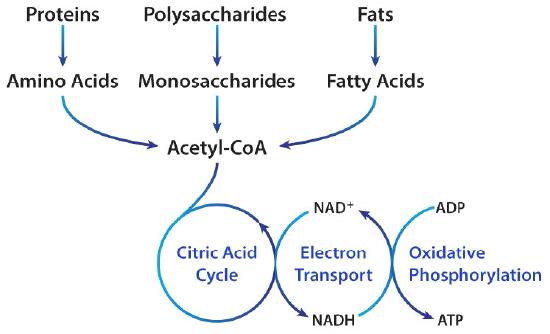
Figure 6.77 – Acetyl-CoA metabolism. Image by Aleia Kim
Acetyl-CoA metabolism
Acetyl-CoA is one of the most “connected” metabolites in biochemistry, appearing in fatty acid oxidation/synthesis, pyruvate oxidation, the citric acid cycle, amino acid anabolism/catabolism, ketone body metabolism, steroid/bile acid synthesis, and (by extension from fatty acid metabolism) prostaglandin synthesis .
These many different metabolic pathways converge at this key molecule, feeding into the cycle to provide organisms energy from a variety of food and stored chemical sources.

