Lipids are a diverse group of molecules that all share the characteristic that at least a portion of them is hydrophobic. Lipids play many roles in cells, including serving as energy storage (fats/oils), constituents of membranes (glycerophospholipids, sphingolipids, cholesterol), hormones (steroids), vitamins (fat soluble), oxygen/ electron carriers (heme), among others. For lipids that are very hydrophobic, such as fats/ oils, movement and storage in the aqueous environment of the body requires special structures. Other, amphipathic lipids, such as glycerophospholipids and sphingolipids spontaneously organize themselves into lipid bilayers when placed in water. Interestingly, major parts of many lipids can be constructed from acetyl-CoA, so they can be made from other nutrients including carbohydrates. That said, some lipids are dietarily essential.
Fatty acids
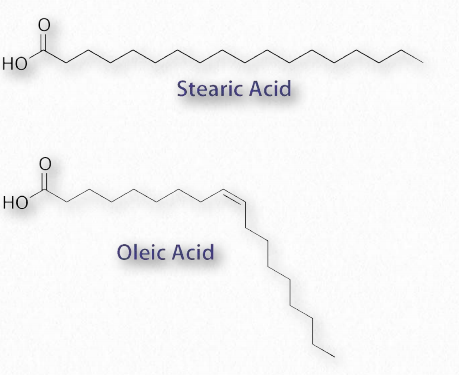
Figure 2.190 – Saturated fatty acid (stearic acid) and unsaturated fatty acid (oleic acid)
The most ubiquitous lipids in cells are the fatty acids. Found in triglycerides and glycerophospholipids, and serving as as membrane anchors for proteins and other biomolecules, fatty acids are important for energy storage, membrane structure, and as precursors of most classes of lipids. Fatty acids, as can be seen from Figure 2.190 are characterized by a polar or charged, hydrophilic head group and a long, hydrocarbon, and thus hydrophobic tail.
Fatty acids with hydrocarbon tails that lack any double bonds are described as saturated, while those with one or more double bonds in their tails are known as unsaturated fatty acids. The effect of double bonds on the fatty acid tail is to introduce a kink, or bend, in the tail, as shown for oleic acid.
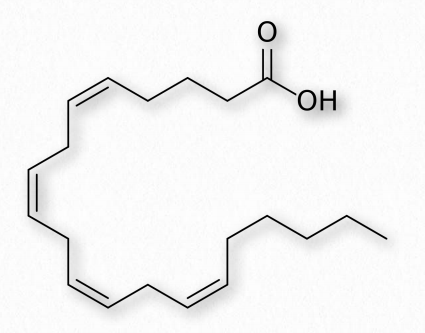
Figure 2.191 – Arachidonic acid – A polyunsaturated fatty acid Wikipedia
The saturated fatty acid named stearic acid, by contrast, has a straight hydrocarbon tail. Figures 2.190-2.194 show the most common saturated and unsaturated fatty acids. Fatty acids with unsaturated tails pack less tightly together, have weaker London forces between them, and thus have a lower melting temperature than those with saturated tails of the same length. Shorter tails also decrease melting temperature. These properties carry over to the fats/oils containing them.
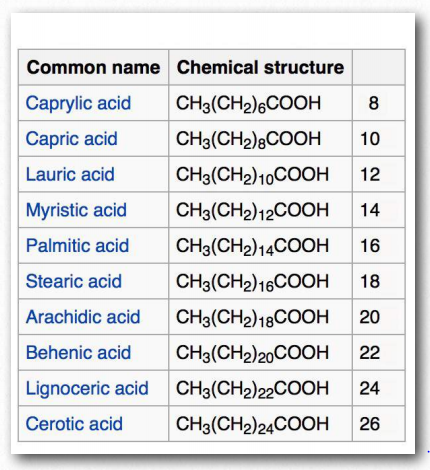
Figure 2.192 – Saturated fatty acids. Number of carbons in right column Wikipedia
Fatty acids with more than one double bond are called polyunsaturated. Plants are excellent sources of unsaturated and polyunsaturated fatty acids. The position of the double bond(s) in fatty acids has important considerations both for their biosynthesis and for their actions in the body. Biochemically, the double bonds found in fatty acids are nearly always in the cis configuration. So-called trans fats arise as a chemical by-product of partial hydrogenation of vegetable oil, and very occasionally naturally.
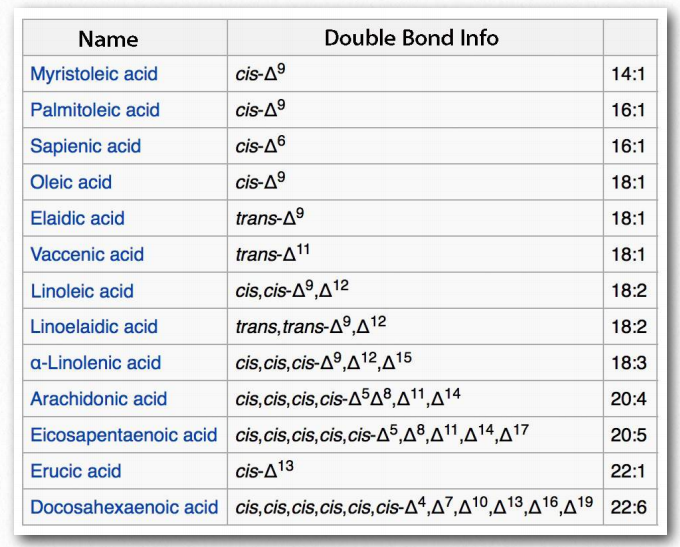
Figure 2.193 – Unsaturated fatty acids. Right column Indicates number of carbons and double bonds Wikipedia
In humans, consumption of trans is associated with higher low density lipoprotein (LDL) levels and lower high density lipoprotein (HDL) levels. Each of these effects is thought to contribute to the risk of developing coronary artery disease.
The most common fatty acids in our body include palmitate, stearate, oleate, linolenate, linoleate, and arachidonate.
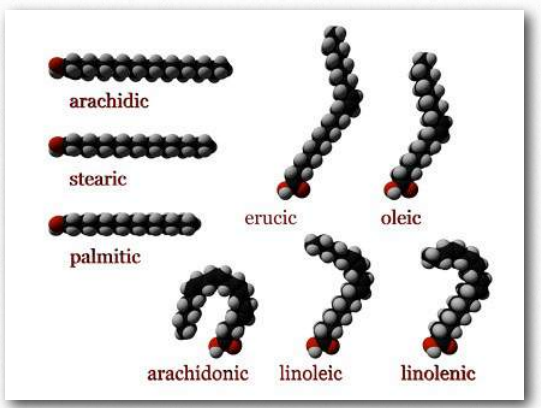
Figure 2.194 – Fatty acid models. Carboxyl end labeled in red Wikipedia
Numbering
Figure 2.195 shows two different systems for locating double bonds in a fatty acid. The ω system counts carbons starting with the methyl end (shown in red) while the Δ system counts from the carboxyl end (shown in blue). For example, an ω-3 (omega 3) fatty acid would have a double bond at the third carbon from the methyl end. In the Δ system, a fatty acid that has a cis double bond at carbon 6, counting from the carboxyl end, would be written as cis-Δ6.
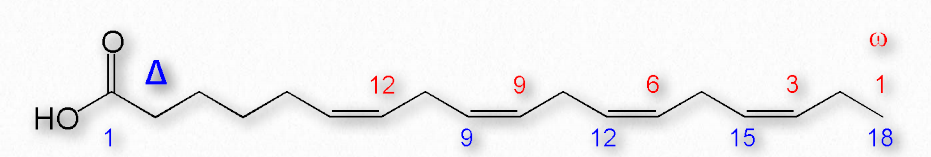
Figure 2.195 – Δ and ω numbering systems for fatty acids Image by Pehr Jacobson
Fatty acids are described as essential fatty acids if they must be in the diet and nonessential fatty acids if the organism can synthesize them. Humans and other animals lack the desaturase enzymes necessary to make double bonds at positions greater than Δ-9, so fatty acids with double bonds beyond this position must be obtained in the diet. Linoleic acid and linolenic acid both fall in this category. Related unsaturated fatty acids can be made from these fatty acids, so the presence of linoleic and linolenic acids in the diet eliminates the need to have all unsaturated fatty acids in the diet. Both linoleic and linolenic acid contain 18 carbons, but linoleic acid is an ω-6 fatty acid, whereas linolenic acid is an ω-3 fatty acid. Notably, ω-6 fatty acids tend to be proinflammatory, whereas ω-3 fatty acids are lesser so.
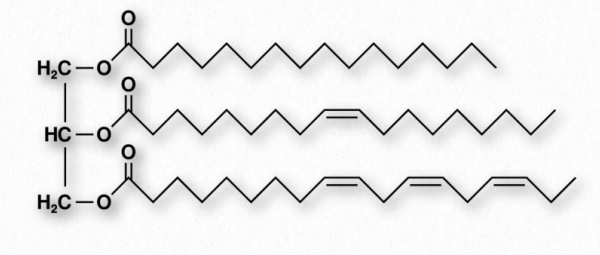
Figure 2.196 – Structure of a triglyceride
Triglycerides
Fats and oils are the primary energy storage forms of animals and are also known as triacylglycerols and triglycerides, since they consist of a glycerol molecule linked via ester bonds to three fatty acids (Figure 2.196). Fats and oils have the same basic structure. We give the name fat to those compounds that are solid at room temperature and the name oil to those that are liquid at room temperature. Note that biological oils are not the same as petroleum oils.
Increasing the amount of unsaturation in a fat decreases its melting temperature. Organisms like fish, which live in cool environments, have fats with more unsaturation. So fish oil from cold-water species, for instance, has more unsaturation and is marketed as a rich source of healthy fats.
Adipocytes
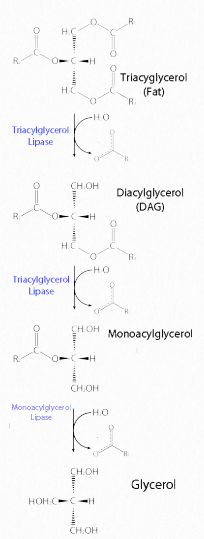
Figure 2.197 – Lipase action on a fat Image by Aleia Kim
Fats are stored in the body in specialized cells known as adipocytes. Enzymes known as lipases release fatty acids from fats by hydrolysis reactions (Figure 2.197). Triacylglycercol lipase (pancreatic – Figure 2.198) is able to cleave the first two fatty acids from the fat. A second enzyme, monoacylglycerol lipase, cleaves the last fatty acid.
Figure 2.198 – Pancreatic lipase
Glycerophospholipids
Glycerophospholipids (phosphoglycerides) are important components of the lipid bilayer of cellular membranes. Phosphoglycerides are structurally related to fats, as both are derived from phosphatidic acid (Figure 2.199). In these structures various groups such as ethanolamine, serine, choline, inositol, and others (Figure 2.200) are linked to the phosphate of phosphatidic acid and then to one carbon of glycerol, via an ester linkage. The second and third positions on the glycerol contain ester links to fatty acid, in the same manner as for triglycerides.
All of these compounds form lipid bilayers in aqueous solution , due to their amphiphilic nature. The phosphate-containing head group is hydrophilic, while the acyl chains from the fatty acids are hydrophobic. These molecules spontaneously form bilayer structures in watery environments with the hydrophilic portions interacting strongly with water, pushing the fatty acyl chains to the interior of the structure.
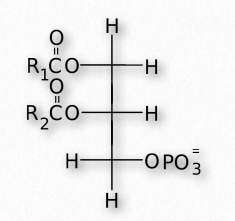
Figure 2.199 – Structure of phosphatidic acid. R1 and R2 are alkyl groups of fatty acids.
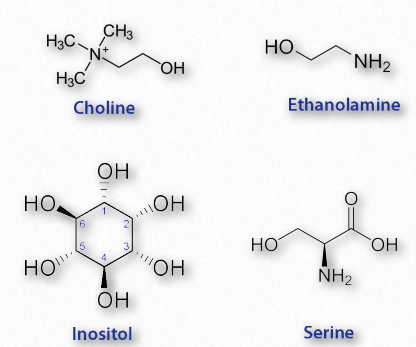
Figure 2.200 – Four common components of phosphatides Wikipedia
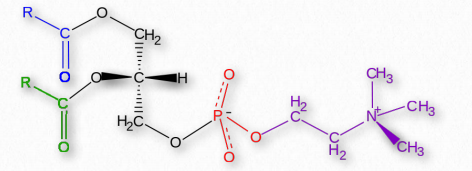
Figure 2.201 – Phosphatidylcholine
Sphingolipids
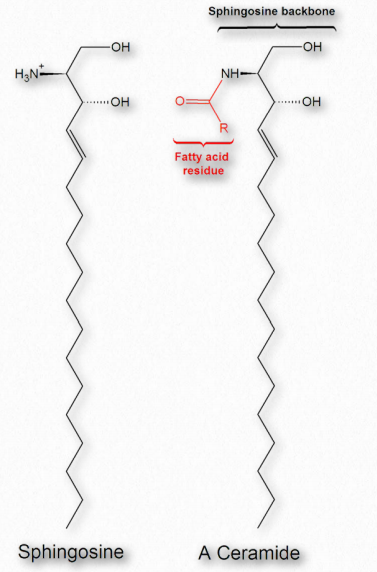
Figure 2.210 – Sphingosine and a ceramide made from it Wikipedia
Fatty acids are also components of a broad class of molecules called sphingolipids. Sphingolipids are structurally similar to glycerophospholipids, though they are synthesized completely independently of them starting with palmitic acid and the amino acid serine. Sphingolipids are named for the amino alcohol known as sphingosine (Figure 2.210), though they are not directly synthesized from it. Figure 2.211 shows the generalized structure of sphingolipids.
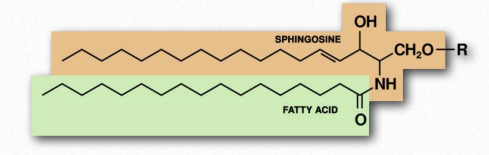
Figure 2.211 Schematic structure of a sphingolipid
If the R-group is a hydrogen, the molecule is called a ceramide. When the R-group is phosphoethanolamine the resulting molecule is sphingomyelin, an important component of the myelin sheath and lipid membranes. If a single, simple sugar is instead added, a cerebroside is created (Figure 2.212). Addition of a complex oligosaccharide creates a ganglioside.
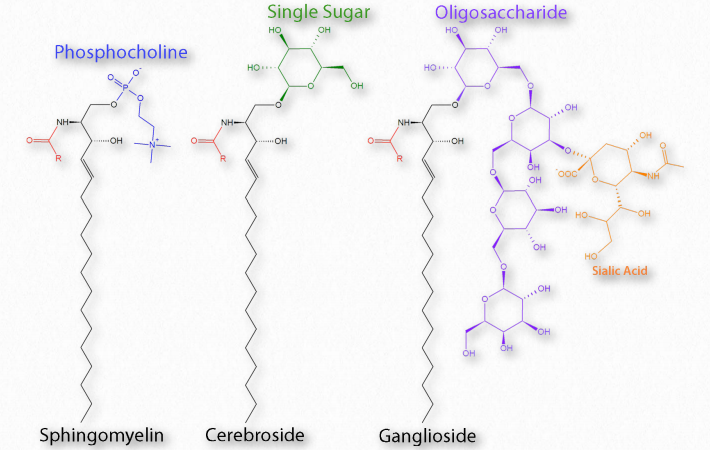
Figure 2.212 – Categories of Sphingolipid Wikipedia
Eicosanoids
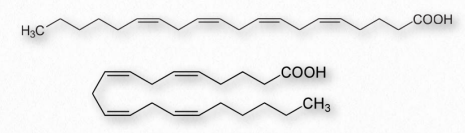
Figure 2.213 – Arachidonic acid drawn as straight (top) and bent (bottom)
Fatty acids made from omega-6 and omega-3 fatty acids include three important fatty acids containing 20 carbons. They include arachidonic acid (an ω-6 fatty acid with four double bonds (Δ-5,8,11,14) – Figure 2.213), eicosapentaenoic acid (an ω-3 fatty acid with five double bonds, and dihomo-γ-linolenic acid (an ω-6 fatty acid with three double bonds). The class of compounds known as eicosanoids is made by oxidation of these compounds. Subclasses include include prostaglandins, prostacyclins, thromboxanes, lipoxins, leukotrienes, and endocannabinoids (Figures 2.214-2.219). Eicosanoids play important roles affecting inflammation, immunity, mood, and behavior.
Prostaglandins
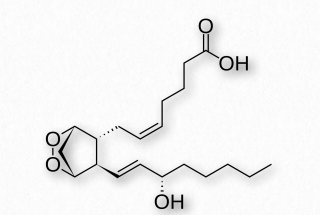
Figure 2.214 – Prostaglandin PGH2
A collection of molecules acting like hormones, prostaglandins are derived from arachidonic acid and have many differing (even conflicting) physiological effects. These include constriction or dilation of vascular smooth muscle cells, induction of labor, regulation of inflammation, and action on the thermoregulatory center of the hypothalamus to induce fever, among others.
Prostaglandins are grouped with the thromboxanes (below) and prostacyclins (below), as prostanoids. The prostanoids, which all contain 20 carbons are a subclass of the eicosanoids. Prostaglandins are found in most tissues of higher organisms. They are autocrine or paracrine compounds produced from essential fatty acids.
Interesting prostaglandins
PGD2 – inhibits hair follicle growth, vasodilator, causes bronchial constriction, higher in lungs of asthmatics than others.
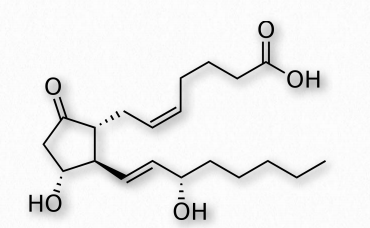
Figure 2.215 Prostaglandin E
PGE2 (Figure 2.215) – exerts effects in labor (soften cervix, uterine contraction), stimulates bone resorption by osteoclasts, induces fever, suppresses T-cell receptor signaling, vasodilator, inhibits release of noradrenalin from sympathetic nerve terminals. It is a potent activator of the Wnt signaling pathway.
A prostaglandin can have opposite effects, depending on which receptor it binds to. Binding of PGE2 to the EP1 receptor causes bronchoconstriction and smooth muscle contraction, whereas binding of the same molecule to the EP2 receptor causes bronchodilation and smooth muscle relaxation.
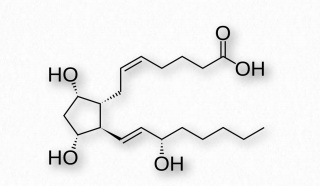
Figure 2.216 – Prostaglandin F2α
PGF2α (Figure 2.216)- uterine contractions, induces labor, bronchoconstriction.
PGI2 – vasodilation, bronchodilation, inhibition of platelet aggregation.
Thromboxanes
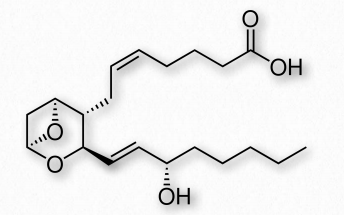
Figure 2.217 Thromboxane A2 2
Thromboxanes play roles in blood clot formation and named for their role in thrombosis. They are potent vasoconstrictors and facilitate platelet aggregation. They are synthesized in platelets, as well. The anti-clotting effects of aspirin have their roots in the inhibition of synthesis of PGH2, which is the precursor of the thromboxanes. The most common thromboxanes are A2 (Figure 2.217) and B2.
Prostacyclin
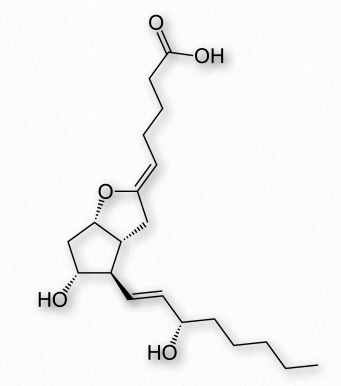
Figure 2.218 – Prostacyclin
Prostacyclin (also known as prostaglandin I2 or PGI2 – Figure 2.218) counters the effects of thromboxanes, inhibiting platelet activation and acting as vasodilators. It is produced from PGH2 by action of the enzyme prostacyclin synthase.
Leukotrienes
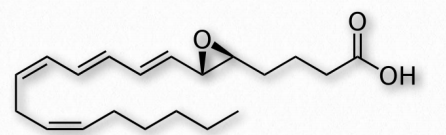
Figure 2.219 – Leukotriene A4 (LTA4)
Another group of eicosanoid compounds are the leukotrienes (Figure 2.219). Like prostaglandins, leukotrienes are made from arachidonic acid. The enzyme catalyzing their formation is a dioxygenase known as arachidonate 5-lipoxygenase. Leukotrienes are involved in regulating immune responses. They are found in leukocytes and other immunocompetent cells, such as neutrophils, monocytes, mast cells, eosinophils, and basophils. Leukotrienes are associated with production of histamines and prostaglandins, which act as mediators of inflammation. Leukotrienes also trigger contractions in the smooth muscles of the bronchioles. When overproduced, they may pay a role in asthma and allergic reactions. Some treatments for asthma aim at inhibiting production or action of leukotrienes.
Cholesterol
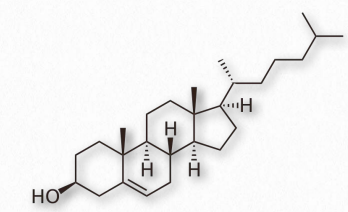
Figure 2.220 – Structure of cholesterol
Arguably, no single biomolecule has generated as much discussion and interest as has cholesterol (Figure 2.220). Certainly, from the perspective of the Nobel Prize committee, no small molecule even comes close, with 13 people having been awarded prizes for work on it. Evidence for cholesterol’s importance comes from the study of brain tissue where it comprises 10-15% of the dry mass.
Membrane flexibility
Figure 2.221 – Sitosterol – A phytosterol
In animal cells, cholesterol provides for membrane flexibility that allows for cellular movement that is in contrast to plant and bacterial cells with fixed structures. Cholesterol is made in many cells of the body, with the liver making the greatest amount. The anabolic pathway leading to synthesis of cholesterol is known as the isoprenoid pathway and branches of it lead to other molecules including other fat-soluble vitamins.

Figure 2.222 – Margarine – A common source of trans fat Wikipedia
Cholesterol is only rarely found in prokaryotes and is found in only trace amounts in plants. Instead, plants synthesize similar compounds called phytosterols (Figure 2.221). On average, the body of a 150 pound adult male makes about 1 gram of cholesterol per day, with a total content of about 35 grams.
Packaging
Figure 2.223 – Cholesterol – Ball and stick model
Cholesterol’s (and other lipids’) hydrophobicity requires special packaging into lipoprotein complexes (called chylomicrons, VLDLs, IDLs, LDLs, and HDLs) for movement in the lymph system and bloodstream. Though cholesterol can be made by cells, they also take it up from the blood supply by absorbing cholesterol-containing LDLs directly in a process called receptor-mediated endocytosis.
Oxidative damage to LDLs can lead to formation of atherosclerotic plaques. This is why cholesterol has gotten such a negative image in the public eye. The liver excretes cholesterol through the bile into the digestive system, but the compound is recycled from there.
Reducing cholesterol levels
Figure 2.224 – Ezetimibe – An inhibitor of cholesterol absorption
Strategies for reducing cholesterol in the body focus primarily on three areas – reducing consumption, reducing endogenous synthesis, and reducing the recycling via the digestive tract. Dietary considerations about consumption of fats are currently debated. Dietary trans fats, though, quite clearly correlate with incidence of coronary heart disease. Consumption of vegetables may provide some assistance with reducing levels of cholesterol recycled in the digestive system, because plant phytosterols compete with cholesterol for reabsorption and when this happens, a greater percentage of cholesterol exits the body in the feces. Drugs related to penicillin are also used to inhibit cholesterol recycling. One of these is ezetimibe, shown in Figure 2.224.
Figure 2.225 – All-trans retinol
Genetic defects in the cholesterol movement system are a cause of the rare disease known as familial hypercholesterolemia in which the blood of afflicted individuals contains dangerously high levels of LDLs. Left untreated, the disease is often fatal in the first 10-20 years of life. While LDLs have received (and deserve) a bad rap, another group of lipoprotein complexes known as the HDLs (high density lipoprotein complexes) are known as “good cholesterol” because their levels correlate with removal of debris (including cholesterol) from arteries and reduce inflammation.
Membrane function
In membranes, cholesterol is important as an insulator for the transmission of signals in nerve tissue. It also helps manage fluidity of membranes over a wide range of temperatures. Stacked in the lipid bilayer, cholesterol decreases a membrane’s fluidity and its permeability to neutral compounds, as well as protons and sodium ions. Cholesterol may play a role in signaling by helping with construction of lipid rafts within the cell membrane.
Lipoprotein complexes and lipid movement in the body
Lipoprotein complexes are combinations of apolipoproteins and lipids bound to them that solubilize fats and other non-polar molecules, such as cholesterol, so they can travel in the bloodstream between various tissues of the body. The apolipoproteins provide the emulsification necessary for this. Lipoprotein complexes are formed in tiny “balls” with the water soluble apolipoproteins on the outside and non-polar lipids, such as fats, cholesteryl esters, and fat soluble vitamins on the inside.
They are categorized by their densities. These include (from highest density to the lowest) high density lipoproteins (HDLs), Low Density Lipoproteins (LDLs), Intermediate Density Lipoproteins (IDLs), Very Low Density Lipoproteins (VLDLs) and the chylomicrons. These particles are synthesized in the liver and small intestines.
Apolipoproteins
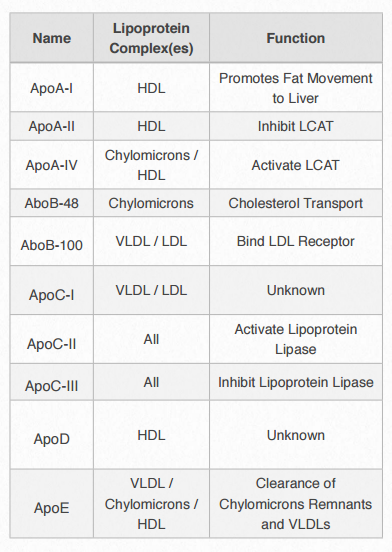
Figure 2.256 – Apolipoproteins
Each lipoprotein complex contain a characteristic set of apolipoproteins, as shown in Figure 2.256. ApoC-II and ApoC-III are notable for their presence in all the lipoprotein complexes and the roles they play in activating (ApoC-II) or inactivating (ApoC-III) lipoprotein lipase. Lipoprotein lipase is a cellular enzyme that catalyzes the breakdown of fat from the complexes. ApoE (see below) is useful for helping the predict the likelihood of the occurrence of Alzheimer’s disease in a patient.
Gene editing
Figure 2.257 – ApoA-I
ApoB-48 and ApoB-100 are interesting in being coded by the same gene, but a unique mRNA sequence editing event occurs that converts one into the other. ApoB-100 is made in the liver, but ApoB-48 is made in the small intestine. The small intestine contains an enzyme that deaminates the cytidine at nucleotide #2153 of the common mRNA. This changes it to a uridine and changes the codon it is in from CAA (codes for glutamine) to UAA (stop codon). The liver does not contain this enzyme and does not make the change in the mRNA. Consequently, a shorter protein is synthesized in the intestine (ApoB-48) than the one that is made in the liver (ApoB-100) using the same gene sequence in the DNA.
ApoE and Alzheimer’s disease
ApoE is a component of the chylomicrons and is also found in brain, macrophages, kidneys, and the spleen. In humans, it is found in three different alleles, E2, E3, and E4. The E4 allele (present at about 14% of the population) is associated with increased likelihood of contracting Alzheimer’s disease. People heterozygous for the allele are 3 times as likely to contract the disease and those homozygous for it are 15 times as likely to do so. It is not known why this gene or allele is linked to the disease. The three alleles differ only slightly in amino acid sequence, but the changes do cause notable structural differences. The E4 allele is associated with increased calcium ion levels and apoptosis after injury. Alzheimer’s disease is associated with accumulation of aggregates of the β- amyloid peptide. ApoE does enhance the proteolytic breakdown of it and the E4 isoform is not as efficient in these reactions as the other isoforms.

