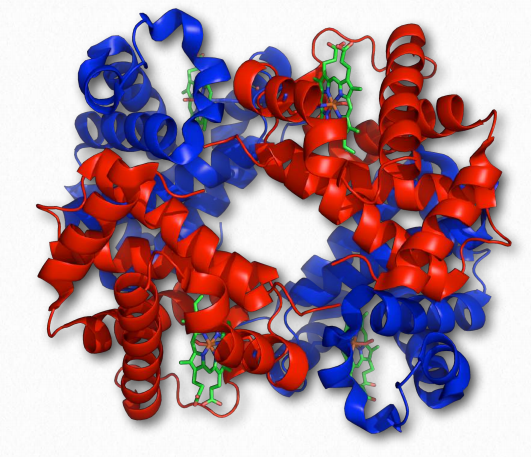
Hemoglobin Wikipedia
Fibrous proteins – secondary structure
Proteins whose cellular or extracellular roles have a strong structural component are composed primarily of primary and second structure, with little folding of the chains. Thus, they have very little tertiary structure and are fibrous in nature. Proteins exhibiting these traits are commonly insoluble in water and are referred to as fibrous proteins (also called scleroproteins). The examples described in this category are found exclusively in animals where they serve roles in flesh, connective tissues and hardened external structures, such as hair. They also contain the three common fibrous protein structures α -helices (keratins), β-strands/sheets (fibroin & elastin) and triple helices (collagen). The fibrous proteins have some commonality of amino acid sequence. Each possesses an abundance of repeating sequences of amino acids with small, non-reactive side groups. Many contain short repeats of sequences, often with glycine.
Keratins

Figure 2.56 – The horns of an impala are composed of keratin Wikipedia
The keratins are a family of related animal proteins that take numerous forms. α-keratins are structural components of the outer layer of human skin and are integral to hair, nails, claws, feathers, beaks, scales, and hooves. Keratins provide strength to tissues, such as the tongue, and over 50 different keratins are encoded in the human genome. At a cellular level, keratins comprise the intermediate filaments of the cytoskeleton. α- keratins primarily contain α-helices, but can also have β-strand/sheet structures. Individual α-helices are often intertwined to form coils of coiled structures and these strands can also be further joined together by disulfide bonds, increasing structural strength considerably. This is particularly relevant for α-keratin in hair, which contains about 14% cysteine. The odor of burned hair and that of the chemicals used to curl/uncurl hair (breaking/re-making disulfide bonds) arise from their sulfurous components. β-keratins are comprised of β-sheets, as their name implies.
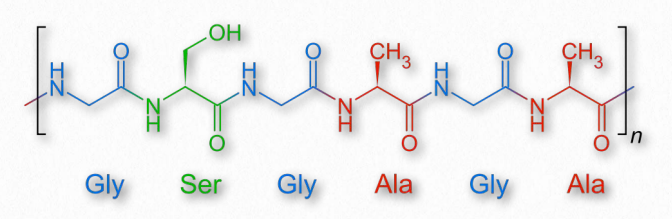
Figure 2.57 – The repeating amino acid sequence of fibroin
Fibroin
An insoluble fibrous protein that is a component of the silk of spiders and the larvae of moths and other insects, fibroin is comprised of antiparallel β-strands tightly packed together to form β- sheets. The primary structure of fibroin is a short repeating sequence with glycine at every other residue (Figure 2.57). The small R-groups of the glycine and alanine in the repeating sequence allows for the tight packing characteristic of the fibers of silk. Wikipedia link HERE Elastin As suggested by its name, elastin is a protein with elastic characteristics that functions in many tissues of the body to allow them to resume their shapes after expanding or contracting. The protein is rich in glycine and proline and can comprise over 50% of the weight of dry, defatted arteries.

Figure 2.58 – Weaving of a silk sari Wikipedia
Elastin
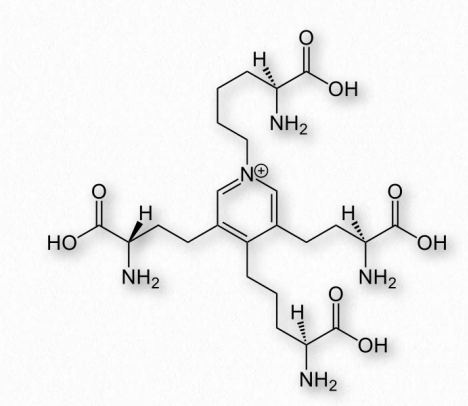
Figure 2.59 – Desmosine Wikipedia
is made by linking tropoelastin proteins together through lysine residues to make a durable complex crosslinked by desmosine. In arteries, elastin helps with pressure wave propagation for facilitating blood flow.
Collagen
Figure 2.60 – Collagen’s triple helix Wikipedia
Collagen is the most abundant protein in mammals, occupying up to a third of the total mass. There are at least 16 types of collagen. Its fibers are a major component of tendons and they are also found abundantly in skin. Collagen is also prominent in cornea, cartilage, bone, blood vessels and the gut.
Collagen’s structure is an example of a helix of helices, being composed of three lefthanded helical chains that each are coiled together in a right-handed fashion to make the collagen fiber (Figure 2.60). Each helix is stretched out more than an α-helix, giving it an extended appearance. On the inside of the triple helical structure, only residues of glycine are found, since the side chains of other amino acids are too bulky. Collagen chains have the repeating structure glycinem-n where m is often proline and n is often hydroxyproline (Figure 2.61).

Figure 2.61 – Repeating sequences in collagen
Collagen is synthesized in a pre-procollagen form. Processing of the pre-procollagen in the endoplasmic reticulum results in glycosylation, removal of the ‘pre’ sequence, and hydroxylation of lysine and proline residues (see below). The hydroxides can form covalent cross-links with each other, strengthening the collagen fibers. As pro-collagen is exported out of the cell, proteases trim it, resulting in a final form of collagen called tropocollagen.
Hydroxylation
Hydroxylation of proline and lysine side chains occurs post-translationally in a reaction catalyzed by prolyl-4-hydroxylase and lysyl-hydroxylase (lysyl oxidase), respectively. The reaction requires vitamin C. Since hydroxylation of these residues is essential for formation of stable triple helices at body temperature, vitamin C deficiency results in weak, unstable collagen and, consequently, weakened connective tissues. It is the cause of the disease known as scurvy. Hydrolyzed collagen is used to make gelatin, which is important in the food industry. collagens. Wikipedia link HERE
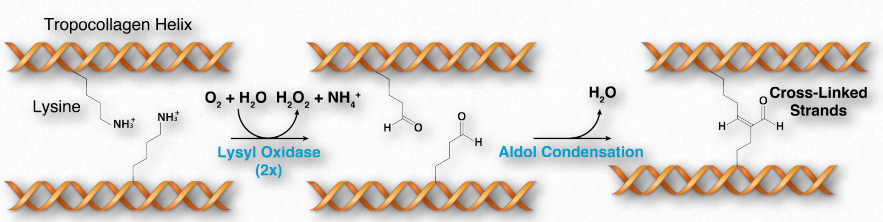
Figure 2.62 – Oxidation and cross-linking of lysine residues in tropocollagen. Only two strands of the triple helix are shown for simplicity Image by Aleia Kim
Lamins
Lamins are fibrous proteins that provide structure in the cell nucleus and play a role in transcription regulation. They are similar to proteins making up the intermediate filaments, but have extra amino acids in one coil of the protein. Lamins help to form the nuclear lamin in the interior of the nuclear envelope and play important roles in assembling and disassembling the latter in the process of mitosis. They also help to position nuclear pores. In the process of mitosis, disassembly of the nuclear envelope is promoted by phosphorylation of lamins by a protein called mitosis promoting factor and assembly is favored by reversing the reaction (dephosphorylation).
Structural domains – tertiary structure
Every globular protein relies on its tertiary structure to perform its function, so rather than trying to find representative proteins for tertiary structure (an almost impossible task!), we focus here on a few elements of tertiary structure that are common to many proteins. These are the structural domains and they differ from the structural motifs of supersecondary structure by being larger (25-500 amino acids), having a conserved amino acid sequence, and a history of evolving and functioning independently of the protein chains they are found in. Structural domains are fundamental units of tertiary structure and are found in more than one protein. A structural domain is selfstabilizing and often folds independently of the rest of the protein chain.
Leucine zipper
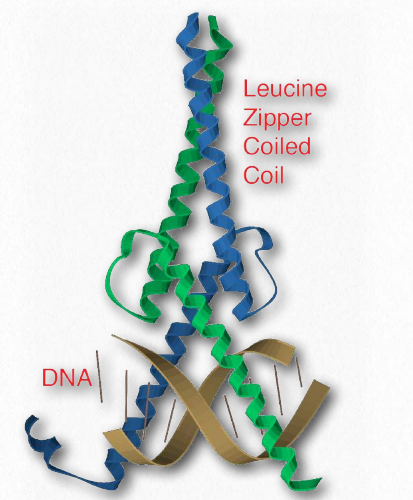
Figure 2.63 – Leucine zipper bound to DNA Wikipedia
A common feature of many eukaryotic DNA binding proteins, leucine zippers are characterized by a repeating set of leucine residues in a protein that interact like a zipper to favor dimerization. Another part of the domain has amino acids (commonly arginine and lysine) that allow it to interact with the DNA double helix (Figure 2.63). Transcription factors that contain leucine zippers include Jun-B, CREB, and AP-1 fos/ jun.
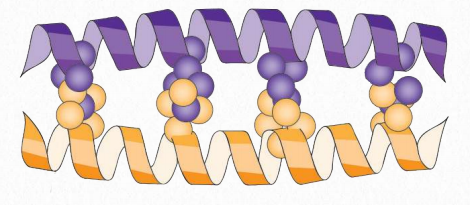
Figure 2.64 – Leucine zipper structure. Leucines are indicated by orange and purple balls. Image by Penelope Irving
Zinc fingers
The shortest structural domains are the zinc fingers, which get their name from the fact that one or more coordinated zinc ions stabilize their finger-like structure. Despite their name, some zinc fingers do not bind zinc. There are many structural domains classified as zinc fingers and these are grouped into different families. Zinc fingers were first identified as components of DNA binding transcription factors, but others are now known to bind RNA, protein, and even lipid structures. Cysteine and histidine side chains commonly play roles in coordinating the zinc.
Src SH2 domain
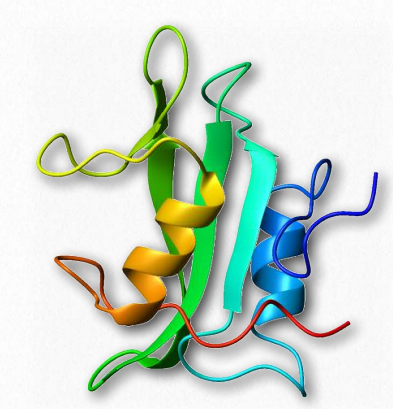
Figure 2.65 – SH2 Domain Wikipedia
The Src oncoprotein contains a conserved SH2structural domain that recognizes and binds phosphorylated tyrosine side chains in other proteins (Figure 2.65). Phosphorylation is a fundamental activity in signaling and phosphorylation of tyrosine and interaction between proteins carrying signals is critically needed for cellular communication. The SH2domain is found in over 100 human proteins.
Helix-turn-helix domain
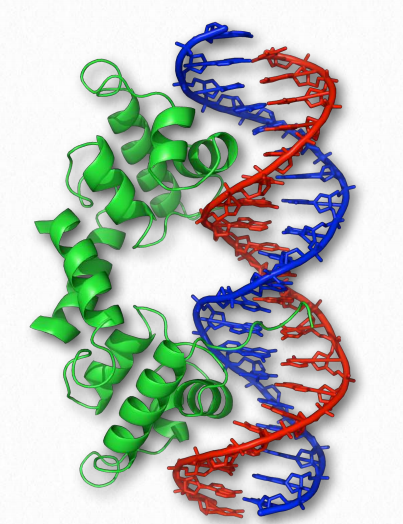
Figure 2.66 – Helix-Turn-Helix Domain of a Protein Bound to DNA Wikipedia
Helix-turn-helix is a common domain found in DNA binding proteins, consisting of two α-helices separated by a small number of amino acids. As seen in Figure 2.66, the helix parts of the structural domain interact with the bases in the major groove of DNA. Individual α-helices in a protein are part of a helix-turn-helix structure, where the turn separates the individual helices.
Pleckstrin homology domain
Pleckstrin Homology (PH) domains are protein domains with important functions in the process of signaling. This arises partly from the affinity for binding phosphorylated inositides, such as PIP2 and PIP3, found in Figure 2.66 – Helix-Turn-Helix Domain of a Protein Bound to DNA Wikipedia Figure 2.65 – SH2 Domain Wikipedia biological membranes. PH domains can also bind to G-proteins and protein kinase C. The domain spans about amino acids and is found in numerous signaling proteins. These include Akt/Rac Serine/ Threonine Protein Kinases, Btk/ltk/Tec tyrosine protein kinases, insulin receptor substrate (IRS-1), Phosphatidylinositolspecific phospholipase C, and several yeast proteins involved in cell cycle regulation.
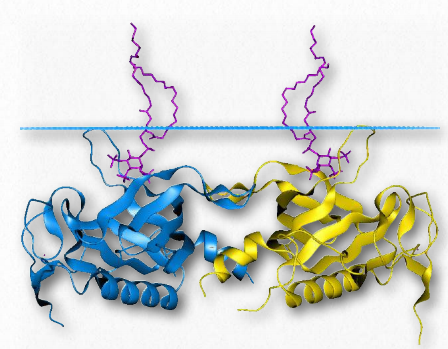
Figure 2.67 – Pleckstrin homology domain of Btk tyrosine protein kinase. The protein is embedded in a membrane (above blue line) Wikipedia
Structural globular proteins
Enzymes catalyze reactions and proteins such as hemoglobin perform important specialized functions. Evolutionary selection has reduced and eliminated waste so that we can be sure every protein in a cell has a function, even though in some cases we may not know what it is. Sometimes the structure of the proFigure 2.68 – Relationship of basement membrane to epithelium, endothelium, and connective tissue tein is its primary function because the structure provides stability, organization, connections other important properties. It is with this in mind that we present the following proteins.
Basement membrane
The basement membrane is a layered extracellular matrix of tissue comprised of protein fibers (type IV collagen) and glycosaminoglycans that separates the epithelium from other tissues (Figure 2.68). More importantly, the basement membrane acts like a glue to hold tissues together. The skin, for example, is anchored to the rest of the body by the basement membrane. Basement membranes provide an interface of interaction between cells and the environment around them, thus facilitating signaling processes. They play roles in differentiation during embryogenesis and also in maintenance of function in adult organisms.
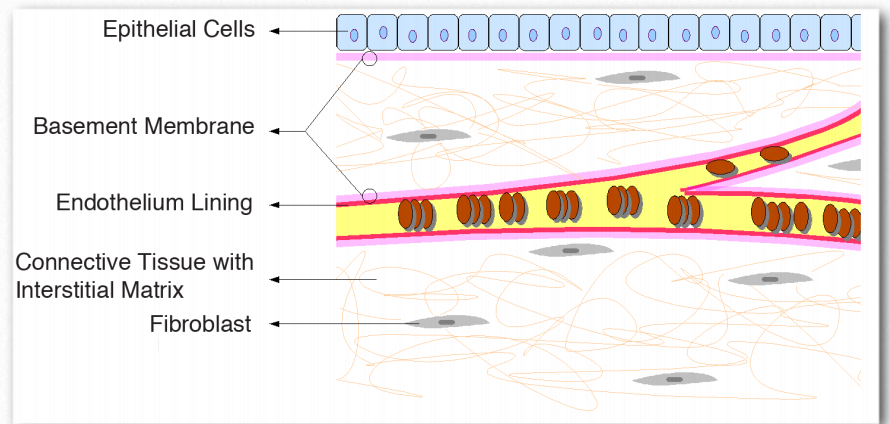
Figure 2.68 – Relationship of basement membrane to epithelium, endothelium, and connective tissue
Actin
Actin is the most abundant globular protein found in most types of eukaryotic cells, comprising as much as 20% of the weight of muscle cells. Similar proteins have been identified in bacteria (MreB) and archaeons (Ta0583). Actin is a monomeric subunit able to polymerize readily into two different types of filaments. Microfilaments are major component of the cytoskeleton and are acted on by myosin in the contraction of muscle cells (See HERE). Actin will be discussed in more detail in the next section HERE.
Intermediate Filaments
Figure 2.69 – Assembly of intermediate filaments
Intermediate filaments are a part of the cytoskeleton in many animal cells and are comprised of over 70 different proteins. They are called intermediate because their size (average diameter = 10 nm) is between that of the microfilaments (7 nm) and the microtubules (25 nm).
The intermediate filament components include fibrous proteins, such as the keratins and the lamins, which are nuclear, as well as cytoplasmic forms. Intermediate filaments give flexibility to cells because of their own physical properties. They can, for example, be stretched to several times of their original length.
Six types
There are six different types of intermediate filaments. Type I and II are acidic or basic and attract each other to make larger filaments. They include epithelial keratins and trichocytic keratins (hair components). Type III proteins include four structural proteins – desmin, GFAP (glial fibrillary acidic protein), peripherin, and vimentin. Type IV also is a grouping of three proteins and one multiprotein structure (neurofilaments). The three proteins are α-internexin, synemin, and syncoilin. Type V intermediate filaments encompass the lamins, which give structure to the nucleus. Phosphorylation of lamins leads to their disassembly and this is important in the process of mitosis. The Type VI category includes only a single protein known as nestin.
Tubulin
A third type of filament found in cells is that of the microbutules. Comprised of a polymer of two units of a globular protein called tubulin, microtubules provide “rails” for motor proteins to move organelles and other “cargo” from one part of a cell to another. Microtubules and tubulin are discussed in more detail HERE.
Vimentin
Vimentin (Figure 2.70) is the most widely distributed protein of the intermediate filaments. It is expressed in fibroblasts, leukocytes, and blood vessel endothelial cells. The protein has a significant role maintaining the position of organelles in the cytoplasm, with attachments to the nucleus, mitochondria, and endoplasmic reticulum (Figure 2.70). Vimentin provides elasticity to cells and resilience that does not arise from the microtubules or microfilaments. Wounded mice that lack the vimentin gene survive, but take longer to heal wounds than wild type mice. Vimentin also controls the movement of cholesterol from lysosomes to the site of esterification. The result is a reduction in the amount of cholesterol stored inside of cells and has implications for adrenal cells, which must have esters of cholesterol.
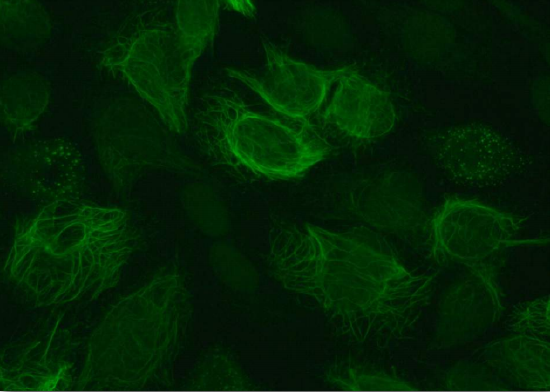
Figure 2.70 – Vimentin in cells Wikipedia
Mucin
Mucins are a group of proteins found in animal epithelial tissue that have many glycosyl residues on them and typically are of high molecular weight (1 to 10 million Da). They are gel-like in their character and are often used for lubrication. Mucus is comprised of mucins. In addition to lubrication, mucins also help to control mineralization, such as bone formation in vertebrate organisms and calcification in echinoderms. They also play roles in the immune system by helping to bind pathogens. Mucins are commonly secreted onto mucosal surfaces (nostrils, eyes, mouth, ears, stomach, genitals, anus) or into fluids, such as saliva. Because of their extensive mucosylation, mucins hold a considerable amount of water (giving them the “slimy” feel) and are resistant to proteolysis.
Vinculin
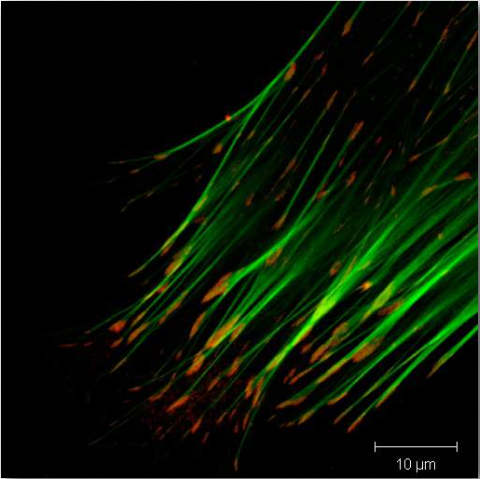
Figure 2.71 – Actin filaments (green) attached to vinculin in focal adhesion (red) Wikipedia
Vinculin (Figure 2.72) is a membrane cytoskeletal protein found in the focal adhesion structures of mammalian cells. It is found at cell-cell and cell-matrix junctions and interacts with integrins, talin, paxillins and F-actin. Vinculin is thought to assist (along with other proteins) in anchoring actin microfilaments to the membrane (Figure 2.71). Binding of vinculin to actin and to talin is regulating by polyphosphoinositides and can be inhibited by acidic phospholipids.
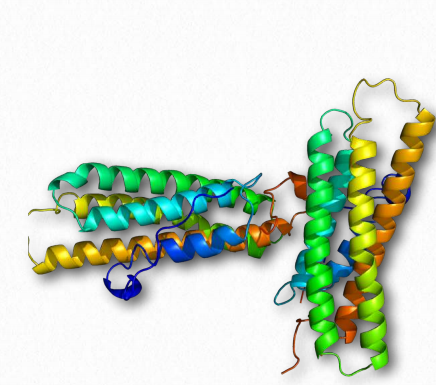
Figure 2.72 – Vinculin Wikipedia
Syndecans
Syndecans are transmembrane proteins that make a single pass with a long amino acid chain (24-25 residues) through plasma membranes and facilitate G proteincoupled receptors’ interaction with Figure 2.71 – Actin filaments (green) attached to vinculin in focal adhesion (red) Wikipedia ligands, such as growth factors, fibronectin, collagens (I, III, and IV) and antithrombin-1. Syndecans typically have 3-5 heparan sulfate and chondroitin sulfate chains attached to them.
Heparan sulfate can be cleaved at the site of a wound and stimulate action of fibroblast growth factor in the healing process. The role of syndecans in cell-cell adhesion is shown in mutant cells lacking syndecan I that do not adhere well to each other. Syndecan 4 is also known to adhere to integrin. Syndecans can also inhibit the spread of tumors by the ability of the syndecan 1 ectodomain to suppress growth of tumor cells without affecting normal epithelial cells.
Defensin
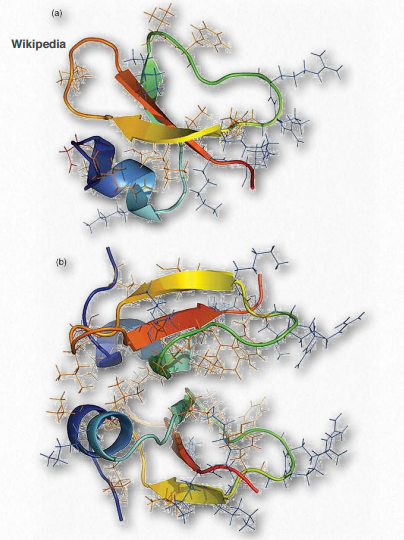
Figure 2.73 – Defensin monomer (top) and dimer (bottom) – Cationic residues in blue, hydrophobic residues in orange, and anionic residues in red Wikipedia
Defensins (Figure 2.73) are a group of small cationic proteins (rich in cysteine residues) that serve as host defense peptides in vertebrate and invertebrate organisms. They protect against infection by various bacteria, fungi, and viruses. Defensins contain between 18 and 45 amino acids with (typically) about 6- 8 cysteine residues. In the immune system, defensins help to kill bacteria engulfed by phagocytosis by epithelial cells and neutrophils. They kill 120 Figure 2.72 – Vinculin Wikipedia bacteria by acting like ionophores – binding the membrane and opening pore-like structures to release ions and nutrients from the cells.
Focal adhesions
In the cell, focal adhesions are structures containing multiple proteins that mechanically link cytoskeletal structures (actin bundles) with the extracellular matrix. They are dynamic, with proteins bringing and leaving with signals regarding the cell cycle, cell motility, and more almost constantly. Focal adhesions serve as anchors and as a signaling hub at cellular locations where integrins bind molecules and where membrane clustering events occur. Over 100 different proteins are found in focal adhesions.
Focal adhesions communicate important messages to cells, acting as sensors to update information about the status of the extracellular matrix, which, in turn, adjusts/ affects their actions. In sedentary cells, they are stabler than in cells in motion because when cells move, focal adhesion contacts are established at the “front” and removed at the rear as motion progresses. This can be very important in white blood cells’ ability to find tissue damage.
Ankyrin
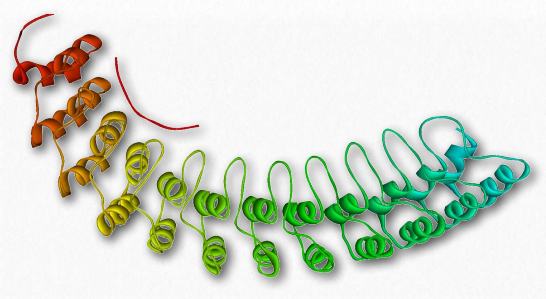
Figure 2.74 – Ankyrin’s membrane-binding domain
Ankyrins (Figure 2.74) are a family of membrane adaptor proteins serving as “anchors” to interconnect integral membrane proteins to the spectrin-actin membrane cytoskeleton. Ankyrins are anchored to the plasma membrane by covalently linked palmitoyl-CoA. They bind to the β subunit of spectrin and at least a dozen groups of integral membrane proteins. The ankyrin proteins contain four functional domains: an N-terminal region with 24 tandem ankyrin repeats, a central spectrin-binding domain, a “death domain” interacting with apoptotic proteins, and a C-terminal regulatory domain that is highly varied significantly among different ankyrins.
Spectrin
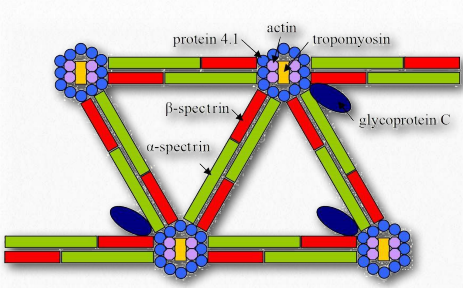
Figure 2.75 – Spectrin and other proteins in the cytoskeleton Wikipedia
Spectrin (Figures 2.75 & 2.76) is a protein of the cellular cytoskeleton that plays an important role in maintaining its structure and the integrity of the plasma membrane. In animals, spectrin gives red blood cells their shape. Spectrin is located inside the inner layer of the eukaryotic plasma membrane where it forms a network of pentagonal or hexagonal arrangements.
Spectrin fibers collect together at junctional complexes of actin and is also attached to ankyrin for stability, as well as numerous integral membrane proteins, such as glycophorin.
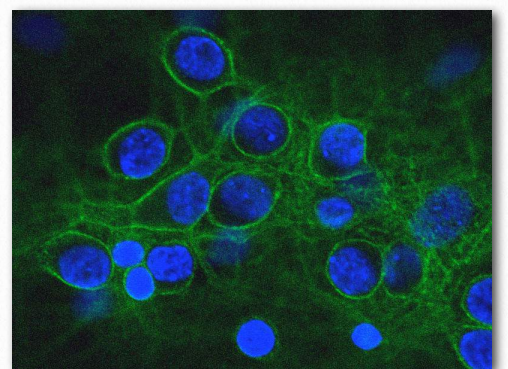
Figure 2.76 – Spectrin (green) and nuclei (Blue) Wikipedia
Integrins
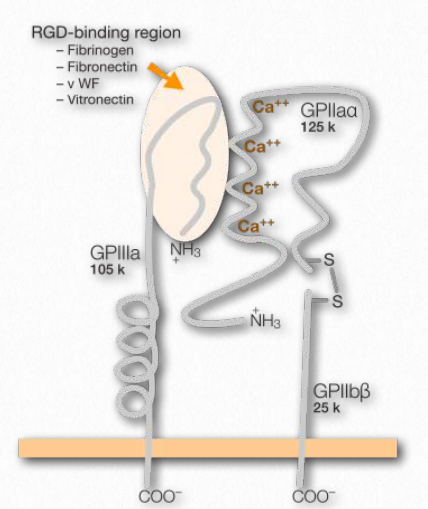
Figure 2.77 – Integrin and its binding site (on top left) Wikipedia
In multicellular organisms, cells need connections, both to each other and to the extracellular matrix. Facilitating these attachments at the cellular end are transmembrane proteins known as integrins (Figure 2.77). Integrins are found in all metazoan cells. Ligands for the integrins include collagen, fibronectin, laminin, and vitronectin. Integrins function not only in attachment, but also in communication, cell migration, virus linkages (adenovirus, for example), and blood clotting. Integrins are able to sense chemical and mechanical signals about the extracellular matrix and move that information to intracellular domains as part of the process of signal transduction. Inside the cells, responses to the signals affect cell shape, regulation of the cell cycle, movement, or changes in other cell receptors in the membrane. The process is dynamic and allows for rapid responses as may be necessary, for example in the process of blood clotting, where the integrin known as GPIbIIIa (on the surface of blood platelets) attaches to fibrin in a clot as it develops.
Integrins work along with other receptors, including immunoglobulins, other cell adhesion molecules, cadherins, selectins, and syndecans. In mammals the proteins have a large number of subunits – 18 α- and 8 β-chains. They are a bridge between its links outside the cell to the extracellular matrix (ECM) and its links inside the cell to the cytoskeleton. Integrins play central role in formation and stability of focal adhesions. These are large molecular complexes arising from clustering of integrin-ECM connections. In the process of cellular movement, integrins at the “front” of the cell (in the direction of the movement), make new attachments to substrate and release connections to substrate in the back of the cell. These latter integrins are then endocytosed and reused.
Integrins also help to modulate signal transduction through tyrosine kinase receptors in the cell membrane by regulating movement of adapters to the plasma membrane. β1c integrin, for example, recruits the Shp2 phosphatase to the insulin growth factor receptor to cause it to become dephosphorylated, thus turning off the signal it communicates. Integrins can also help to recruit signaling molecules inside of the cell to activated tyrosine kinases to help them to communicate their signals.
Cadherins
Figure 2.78 – Extracellular ectodomain of a cadherin
Cadherins (Figure 2.78) constitute a type-1 class of transmembrane proteins playing important roles in cell adhesion. They require calcium ions to function, forming adherens junctions that hold tissues together (See Figure 2.69). Cells of a specific cadherin type will preferentially cluster with each other in preference to associating with cells containing a different cadherin type. Caderins are both receptors and places for ligands to attach. They assist in the proper positioning of cells in development, separation of different tissue layers, and cell migration.
Selectins
Figure 2.79 – Selectin bound to a sugar Wikipedia
Selectins (Figure 2.79) are cell adhesion glycoproteins that bind to sugar molecules. As such, they are a type of lectin – proteins that bind sugar polymers (see HERE also). All selectins have an N-terminal calcium-dependent lectin domain, a single transmembrane domain, and an intracellular cytoplasmic tail.
There are three different types of selectins, 1) E-selectin (endothelial); 2) L (lymphocytic; and 3) P (platelets and endothelial cells. Selectins function in lymphocyte homing (adhesion of blood lymphocytes to cells in lymphoid organs), in inflammation processes, and in cancer metastasis. Near the site of inflammation, P-selectin on the surface of blood capillary cells interacts with glycoproteins on leukocyte cell surfaces. This has the effect of slowing the movement of the leukocyte. At the target site of inflammation, E- selectin on the endothelial cells of the blood vessel and L-selectin on the surface of the leukocyte bind to their respective carbohydrates, stopping the leukocyte movement. The leukocyte then crosses the wall of the capillary and begins the immune response. Selectins are involved in the inflammatory processes of asthma, psoriasis, multiple scleroris, and rheumatoid arthritis.
Laminins
Laminins are extracellular matrix glycoproteins that a major components of the basal lamina and affect cell differentiation, migration, and adhesion. They are secreted into the extracellular matrix where they are incorporated and are essential for tissue maintenance and survival. When laminins are defective, muscles may not form properly and give rise to muscular dystrophy.
Laminins are associated with fibronectin, entactin, and perlecan proteins in type IV collagen networks and bind to integrin receptors in the plasma membrane. As a consequence, laminins contribute to cellular attachment, differentiation, shape, and movement. The proteins are trimeric in structure, having one α-chain, a β-chain, and a γ-chain. Fifteen combinations of different chains are known.
Vitronectin
Vitronectin is a glycoprotein (75kDa) found in blood serum (platelets), the extracellular matrix, and in bone. It promotes the process of cell adhesion and spreading and binds to several protease inhibitors (serpins). It is secreted from cells and is believed to play roles in blood clotting and the malignancy of tumors. One domain of vitronectin binds to plasminogen activator inhibitor and acts to stabilize it. Another domain of the protein binds to cellular integrin proteins, such as the vitronectin receptor that anchors cells to the extracellular matrix.
Catenins
Catenins are a family of proteins interacting with cadherin proteins in cell adhesion (Figure 2.69). Four main types of catenins are known, α-, β-, γ-, and δ-catenin. Catenins play roles in cellular organization before development occurs and help to regulate cellular growth. α-catenin and β-catenin are found at adherens junctions with cadherin and help cells to maintain epithelial layers. Cadherins are connected to actin filaments of the cytoskeleton and catenins play the critical role. Catenins are important for the process whereby cellular division is inhibited when cells come in contact with each other (contact inhibition).
When catenin genes are mutated, cadherin cell adhesions can disappear and tumorigenesis may result. Catenins have been found to be associated with colorectal and numerous other forms of cancer.
Glycophorins
Figure 2.80 – Glycophorin a
All of the membrane proteins described so far are notable for the connections they make to other proteins and cellular structures. Some membrane proteins, though, are designed to reduce cellular connections to proteins of other cells. This is particularly important for blood cells where “stickiness” is undesirable except where clotting is concerned.
Glycophorins (Figure 2.80) are membrane-spanning sialoglycoproteins of red blood cells. They are heavily glycosylated (60%).and rich in sialic acid, giving the cells a very hydrophilic (and negatively charged) coat, which enables them to circulate in the bloodstream without adhering to other cells or the vessel walls.
Five glycophorins have been identified – four (A,B,C,and D) from isolated membranes and a fifth form (E) from coding in the human genome. The proteins are abundant, forming about 2% of the total membrane proteins in these cells. Glycophorins have important roles in regulating RBC membrane mechanical properties and shape. Because some glycophorins can be expressed in various nonerythroid tissues (particularly Glycophorin C), the importance of their interactions with the membrane skeleton may have a considerable biological significance.
Cooperativity and allosterism – quaternary structure
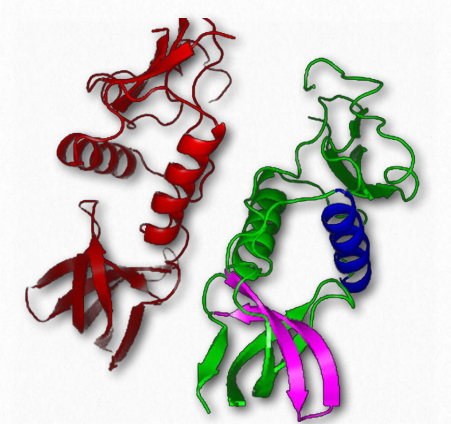
Figure 2.81 – Two polypeptide units of a protein interact in quaternary structure Wikipedia
Quaternary structure, of course describes the interactions of individual subunits of a multi-subunit protein (Figure 2.81). The result of these interactions can give rise to important biological phenomena, such as cooperative binding of substrates to a protein and allosteric effects on the action of an enzyme.
Allosteric effects can occur by a series of mechanisms, but a common feature is that binding of an effector to an enzyme subunit causes (or locks) the enzyme in either a Tstate (less activity) or an R-state (more activity). Effectors can be enzyme substrates (homotropic effectors) or non-substrates (heterotropic effectors). Allosterism will be covered in more depth in the Catalysis chapter HERE.
We begin our consideration of quaternary structure with a discussion of cooperativity, how it arises in the multi-subunit protein hemoglobin and how its properties contrast with those of the related, single subunit protein myoglobin.
Cooperativity
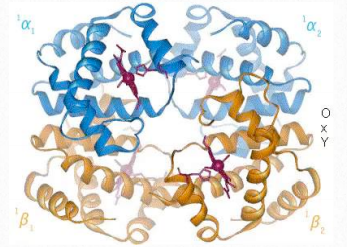
Cooperativity is defined as the phenomenon where binding of one ligand molecule by a protein favors the binding of additional molecules of the same type. Hemoglobin, for example, exhibits cooperativity when the binding of an oxygen molecule by the iron of the heme group in one of the four subunits causes a slight conformation change in the subunit. This happens because the heme iron is attached to a histidine side chain and binding of oxygen ‘lifts’ the iron along with the histidine ring (also known as the imidazole ring).
Movie 2.3 – Hemoglobin’s structural changes on binding oxygen Wikipedia
Since each hemoglobin subunit interacts with and influences the other subunits, they too are induced to change shape slightly when the first subunit binds to oxygen (a transition described as going from the T-state to the R-state). These shape changes favor each of the remaining subunits binding oxygen, as well. This is very important in the lungs where oxygen is picked up by hemoglobin, because the binding of the first oxygen molecule facilitates the rapid uptake of more oxygen molecules. In the tissues, where the oxygen concentration is lower, the oxygen leaves hemoglobin and the proteins flips from the R-state back to the Tstate.
CO2 transport
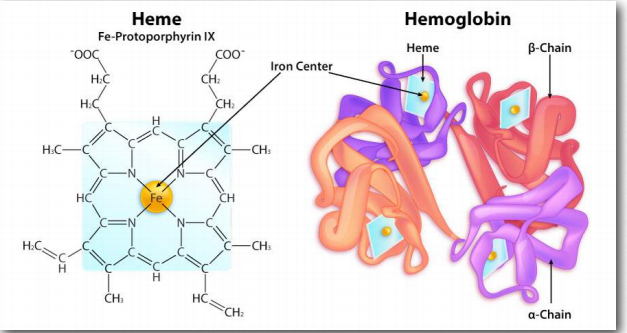
Figure 2.82 – Heme structure within hemoglobin Image by Aleia Kim
Cooperativity is only one of many fascinating structural aspects of hemoglobin that help the body to receive oxygen where it is needed and pick it up where it is abundant. Hemoglobin also assists in the transport of the product of cellular respiration (carbon dioxide) from the tissues producing it to the lungs where it is exhaled. Like the binding of oxygen to hemoglobin, binding of other molecules to hemoglobin affects its affinity for oxygen. The effect is particularly pronounced when comparing the oxygen binding characteristics of hemoglobin’s four subunits with the oxygen binding of the related protein myoglobin’s single subunit (Figure 2.83).
Different oxygen binding
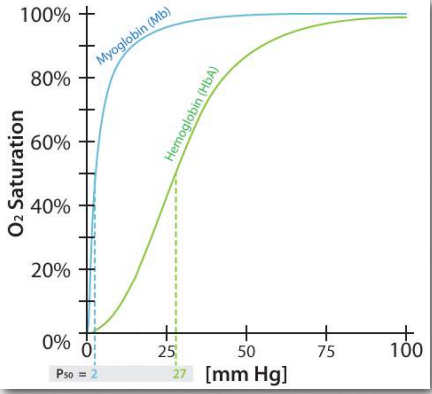
Figure 2.83 – Oxygen binding affinities for myoglobin and hemoglobin Figure by Aleia Kim
Like hemoglobin, myoglobin contains an iron in a heme group that binds to oxygen. The structure of the globin protein in myoglobin is very similar to the structure of the globins in hemoglobin and hemoglobin is thought to have evolved from myoglobin in evolutionary history. As seen in Figure 2.83, the binding curve of hemoglobin for oxygen is S-shaped (sigmoidal), whereas the binding curve for myoglobin is hyperbolic. What this tells us is that hemoglobin’s affinity for oxygen is low at a low concentration oxygen, but increases as the oxygen concentration increases. Since myoglobin very quickly saturates with oxygen, even under low oxygen concentrations, it says that its affinity for oxygen is high and doesn’t change.
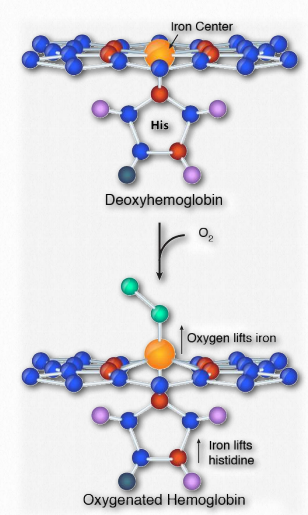
Figure 2.84 – Binding of oxygen at the heme center of hemoglobin Image by Aleia Kim
Because myoglobin has only a single subunit, binding of oxygen by that subunit can’t affect any other subunits, since there are no other subunits to affect. Consequently, cooperativity requires more than one subunit. Therefore, hemoglobin can exhibit cooperativity, but myoglobin can’t. It is worth noting that simply having multiple subunits does not mean cooperativity will exist. Hemoglobin is one protein that exhibits the characteristic, but many multisubunit proteins do not.
Interactive 2.2 – Hemoglobin in the presence (top) and absence (bottom) of oxygen
Storage vs. delivery
The lack of ability of myoglobin to adjust its affinity for oxygen according to the oxygen concentration (low affinity at low oxygen concentration, such as in tissues and high affinity at high oxygen concentration, such as in the lungs) means it is better suited for storing oxygen than for delivering it according to the varying oxygen needs of and animal body. As we shall see, besides cooperativity, hemoglobin has other structural features that allow it to deliver oxygen precisely where it is needed most in the body.
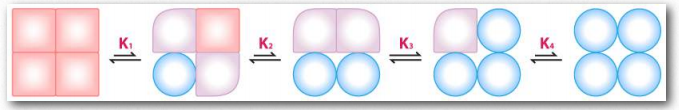
Figure 2.85 – Sequential model of binding. The sequential model is one way to explain hemoglobin’s cooperativity. Squares represent no oxygen bound. Circles represent subunits bound with oxygen and rounded subunits correspond to units whose affinity for oxygen increases by interacting with a subunit that has bound oxygen. Image by Aleia Kim 131
Bohr effect
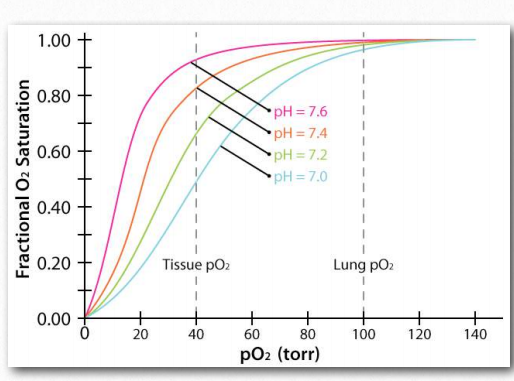
Figure 2.86 – The Bohr effect with respect to pH changes Image by Aleia Kim
The Bohr Effect was first described over 100 years ago by Christian Bohr, father of the famous physicist, Niels Bohr. Shown graphically (Figures 2.86, 2.87, and 2.88), the observed effect is that hemoglobin’s affinity for oxygen decreases as the pH decreases and as the concentration of carbon dioxide increases. Binding of the protons and carbon dioxide by amino Figure 2.85 – Sequential model of binding. The sequential model is one way to explain hemoglobin’s cooperativity. Squares represent no oxygen bound. Circles represent subunits bound with oxygen and rounded subunits correspond to units whose affinity for oxygen increases by interacting with a subunit that has bound oxygen. Image by Aleia Kim acid side chains in the globin proteins helps to facilitate structural changes in them. Most commonly, the amino acid affected by protons is histidine #146 of the β strands. When this happens, the ionized histidine can form an ionic bond with the side chain of aspartic acid #94, which has the effect of stabilizing the T-state (reduced oxygen binding state) and releasing oxygen. Other histidines and the amine of the amino terminal amino acids in the α-chains are also binding sites for protons.
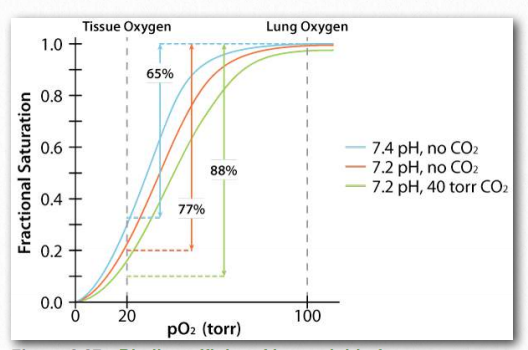
Figure 2.87 – Binding affinity of hemoglobin for oxygen under different conditions Image by Aleia Kim
2,3-BPG
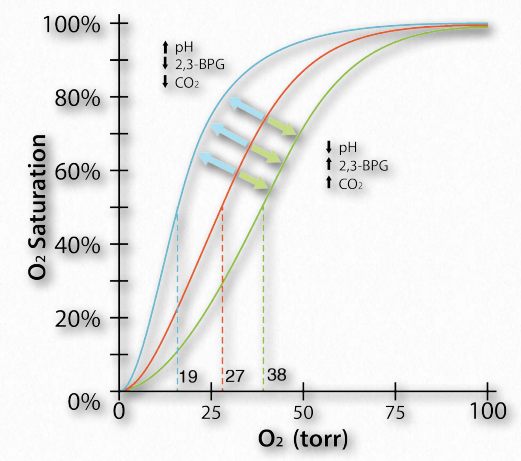
Figure 2.88 – The Bohr effect physiologically – oxygen binding curves for resting muscle (blue), active muscle (green) and reference muscle (orange) with respect to pH, 2,3-BPG, and CO2 Image by Aleia Kim
Another molecule favoring the release of oxygen by hemoglobin is 2,3- bisphosphoglycerate (also called 2,3-BPG or just BPG – Figure 2.89). Like protons and carbon dioxide, 2,3-BPG is produced by actively respiring tissues, as a byproduct of glucose metabolism. The 2,3-BPG mole cule fits into the ‘hole of the donut’ of adult hemoglobin (Figure 2.89). Such binding of 2,3-BPG favors the T-state (tight – low oxygen binding) of hemoglobin, which has a reduced affinity for oxygen. In the absence of 2,3-BPG, hemoglobin can more easily exist in the R-state (relaxed – higher oxygen binding), which has a high affinity for oxygen.
Smokers
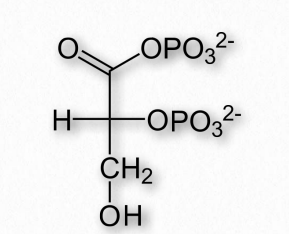
Figure 2.89 – The structure of 2,3 bisphosphoglycerate (2,3-BPG)
Notably, the blood of smokers is higher in the concentration of 2,3-BPG than non-smokers, so more of their hemoglobin remains in the T-state and thus the oxygen carrying capacity of smokers is lower than non-smokers.Another reason why smokers’ oxygen carrying capacity is lower than that of non-smokers is that cigarette smoke contains carbon monoxide and this molecule, which has almost identical dimensions to molecular oxygen, effectively outcompetes with oxygen for binding to the iron atom of heme (Figure 2.90). Part of carbon monoxide’s toxicity is due to its ability to bind hemoglobin and prevent oxygen from binding.
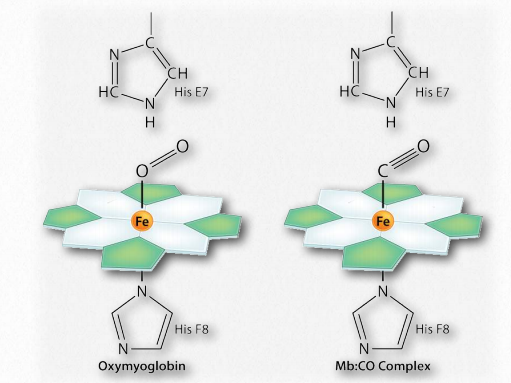
Figure 2.90 – Binding of oxygen (left) and carbon monoxide (right) by a heme group of hemoglobin Image by Aleia Kim
Carbon dioxide
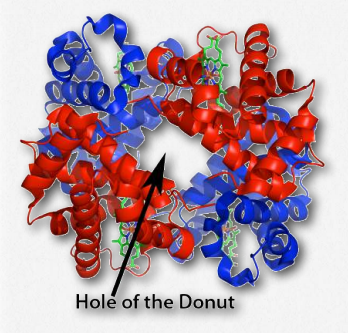
Figure 2.91 – Hemoglobin’s hole of the donut for binding 2,3-BPG Wikipedia
Carbon dioxide binds to form a carbamate when binding the α-amine of each globin chain. The process of forming this structure releases a proton, which helps to further enhance the Bohr effect. Physiologically, the binding of CO2 and H+ has significance because actively respiring tissues (such as contracting muscles) require oxygen and release protons and carbon dioxide. The higher the concentration of protons and carbon dioxide, the more oxygen is released to feed the tissues that need it most.
About 40% of the released protons and about 20% of the carbon dioxide are carried back to the lungs by hemoglobin. The remainder travel as part of the bicarbonate buffering system or as dissolved CO2. In the lungs, the process reverses itself. The lungs have a higher pH than respiring tissues, so protons are released from hemoglobin and CO2 too is freed to be exhaled.
Fetal hemoglobin
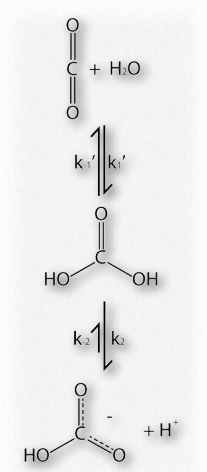
Figure 2.92 – Formation of bicarbonate from CO2 in blood
Adult hemoglobin releases oxygen when it binds 2,3- BPG. This is in contrast to fetal hemoglobin, which has a slightly different configuration (α2γ2) than adult hemoglobin (α2β2). Fetal hemoglobin has a greater affinity for oxygen than maternal hemoglobin, allowing the fetus to obtain oxygen effectively from the mother’s blood. Part of the reason for fetal hemoglobin’s greater affinity for oxygen is that it doesn’t bind 2,3-BPG. Consequently, fetal hemoglobin remains in the R-state much more than adult hemoglobin and because of this, fetal hemoglobin has greater affinity for oxygen than adult hemoglobin and can take oxygen away from adult hemoglobin. Thus, the fetus can get oxygen from the mother.
Figure 2.93 – Comparison of oxygen binding of myoglobin (blue), fetal hemoglobin (orange), and adult hemoglobin (green) Image by Aleia Kim
Sickle cell disease
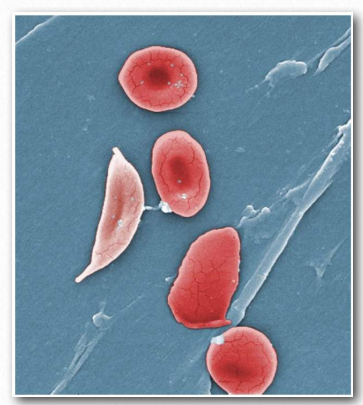
Figure 2.94 – Four normal red blood cells (right) and one sickled red blood cell (left) Wikipedia
Mutations to the globin genes coding for hemoglobin can sometimes have deleterious consequences. Sickle cell disease (also called sickle cell anemia) is a genetically transmitted disease that arises from such mutations. There are different forms of the disease. It is a recessive trait, meaning that to be afflicted with it, an individual must inherit two copies of the mutated gene.
Figure 2.95 – Movement of blood in capillaries. Top – normal red blood cells. Bottom – sickled red blood cells
The predominant form of hemoglobin in adults is hemoglobin A, designated HbA (two α chains and two β chains). The mutant form is known as HbS. The most common mutation is an A to T mutation in the middle of the codon for the seventh amino acid (some counting schemes call it the sixth amino acid) of the β-chain. This results in conversion of a GAG codon to GTG and thus changes the amino acid specified at that position from a glutamic acid to a valine. This minor change places a small hydrophobic patch of amino acids on the surface of the β-globin chains.
Polymerization
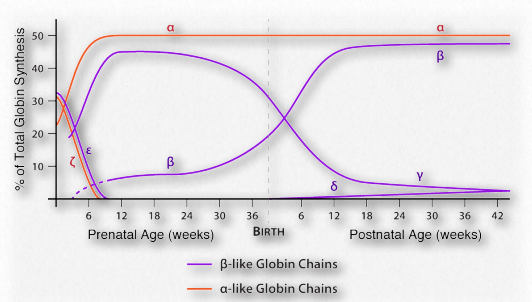
Figure 2.96 – Pattern of expression of six different globins of hemoglobin – α,β,γ,ε,δ, and ζ Image by Aleia Kim
Under conditions of low oxygen, these hydrophobic patches will associate with each other to make long polymers of hemoglobin molecules. The result is that the red blood cells containing them will change shape from being rounded to forming the shape of a sickle (Figure 2.94). Rounded red blood cells readily make it through tiny capillaries, but sickleshaped cells do not.
Worse, they block the flow of other blood cells. Tissues where these blockages occur are already low in oxygen, so stopping the flow of blood through them causes them to go quickly anaerobic, causing pain and, in some cases, death of tissue. In severe circumstances, sickled red blood cells death may result. The disease is referred to as an anemia because the sickling of the red blood cells targets them for removal by the blood monitoring system of the body, so a person with the disease has chronically reduced numbers of red blood cells.
Heterozygote advantage
Interestingly, there appears to be a selective advantage to people who are heterozygous for the disease in areas where malaria is prominent. Heterozygotes do not suffer obvious ill effects of the disease, but their red blood cells appear to be more susceptible to rupture when infected. As a consequence, the parasite gets less of a chance to reproduce and the infected person has a greater chance of survival.
The protective effect of the mutant gene, though, does not extend to people who suffer the full blown disease (homozygotes for the mutant gene). Treatments for the disease include transfusion, pain management, and avoidance of heavy exertion. The drug hydroxyurea has been linked to reduction in number and severity of attacks, as well as an increase in survival time1,2. It appears to work by reactivating expression of the fetal hemoglobin gene, which typically is not synthesized to any significant extent normally after about 6 weeks of age.
Oxygen binding
Animals have needs for oxygen that differ from all other organisms. Oxygen, of course, is the terminal electron acceptor in animals and is necessary for electron transport to work. When electron transport is functioning, ATP generation by cells is many times more efficient than when it is absent. Since abundant ATP is essential for muscular contraction and animals move around a lot – to catch prey, to exercise, to escape danger, etc., having an abundant supply of oxygen is important.
This is particularly a concern deep inside tissues where diffusion of oxygen alone (as occurs in insects) does not deliver sufficient quantities necessary for long term survival. The issue is not a problem for plants since, for the most part, their motions are largely related to growth and thus don’t have rapidly changing needs/demands for oxygen that animals have. Unicellular organisms have a variety of mechanisms for obtaining oxygen and surviving without it. Two other important oxygen binding proteins besides hemoglobin are myoglobin and hemocyanin.
Myoglobin
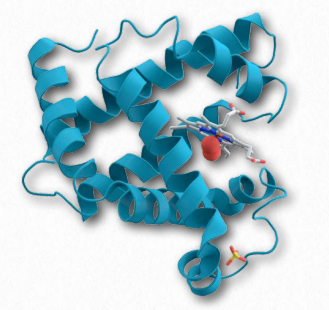
Figure 2.97 – Myoglobin bound to oxygen
Myoglobin is the primary oxygen-storage protein found in animal muscle tissues. In contrast to hemoglobin, which circulates throughout the body, myoglobin protein is only found in muscle tissue and appears in the blood only after injury. Like hemoglobin, myoglobin binds oxygen at a prosthetic heme group it contains.
The red color of meat arises from the heme of myoglobin and the browning of meat by cooking it comes from oxidation of the ferrous (Fe++) ion of myoglobin’s heme to the ferric (Fe+++) ion via oxidation in the cooking process. As meat sits in our atmosphere (an oxygen-rich environment), oxidation of Fe++ to Fe+++ occurs, leaving the brown color noted above. If meat is stored in a carbon monoxide (CO) environment, CO binds to the heme group and reduces the amount of oxidation, keeping meat looking red for a longer period of time.
High affinity
Myoglobin (Figure 2.97) displays higher affinity for oxygen at low oxygen concentrations than hemoglobin and is therefore able to absorb oxygen delivered by hemoglobin under these conditions. Myoglobin’s high affinity for oxygen makes it better suited for oxygen storage than delivery. The protein exists as a single subunit of globin (in contrast to hemoglobin, which contains four subunits) and is related to the subunits found in hemoglobin. Mammals that dive deeply in the ocean, such as whales and seals, have muscles with particularly high abundance of myoglobin. When oxygen concentration in muscles falls to low levels, myoglobin releases its oxygen, thus functioning as an oxygen “battery” that delivers oxygen fuel when needed and holding onto it under all other conditions. Myoglobin holds the distinction of being the first protein for which the 3D structure was determined by X-ray crystallography by John Kendrew in 1958, an achievement for which he later won the Nobel Prize.
Figure 2.98 – Oxygen bound at heme of myoglobin Wikipedia
Hemocyanin
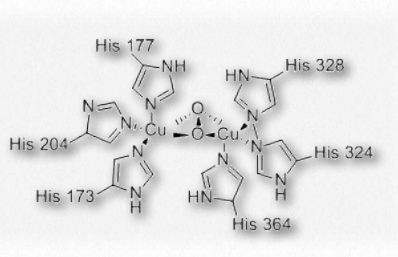
Figure 2.99 – Oxygen binding in hemocyanin Wikipedia
Hemocyanin is the protein transporting oxygen in the bodies of molluscs and arthropods. It is a coppercontaining protein found not within blood cells of these organisms, but rather is suspended in the circulating hemolymph they possess. The oxygen binding site of hemocyanin contains a pair of copper(I) cations directly coordinated to the protein by the imidazole rings of six histidine side chains.

Figure 2.100 – Hemocyanin (purple) in a red rock crab Wikipedia
Most, but not all hemocyanins bind oxygen non-cooperatively and are less efficient than hemoglobin at transporting oxygen. Notably, the hemocyanins of horseshoe crabs and some other arthropods do, in fact, bind oxygen cooperatively. Hemocyanin contains many subunit proteins, each with two copper atoms that can bind one oxygen molecule (O2). Subunit proteins have atomic masses of about 75 kilodaltons (kDa). These may be arranged in dimers or hexamers depending on species. Superstructures comprised of dimer or hexamer complexes are arranged in chains or clusters and have molecular weights of over 1500 kDa.

