Apart from their ability to greatly speed the rates of chemical reactions in cells, enzymes have another property that makes them valuable. This property is that their activity can be regulated, allowing them to be activated and inactivated, as necessary. This is tremendously important in maintaining homeostasis, permitting cells to respond in controlled ways to changes in both internal and external conditions.
Inhibition of specific enzymes by drugs can also be medically useful. Understanding the mechanisms that control enzyme activity is, therefore, of considerable importance.
Inhibition
We will first discuss four types of enzyme inhibition – competitive, non-competitive, uncompetitive, and suicide inhibition. Of these, the first three types are reversible. The last one, suicide inhibition, is not.
Competitive inhibition
Probably the easiest type of enzyme inhibition to understand is competitive inhibition. It is the one most commonly exploited pharmaceutically. Molecules that are competitive inhibitors of enzymes resemble one of the normal substrates of an enzyme. An example is methotrexate, which resembles the folate substrate of the enzyme named dihydrofolate reductase (DHFR). This enzyme normally catalyzes the chemical reduction of folate, an important reaction in the metabolism of nucleotides.
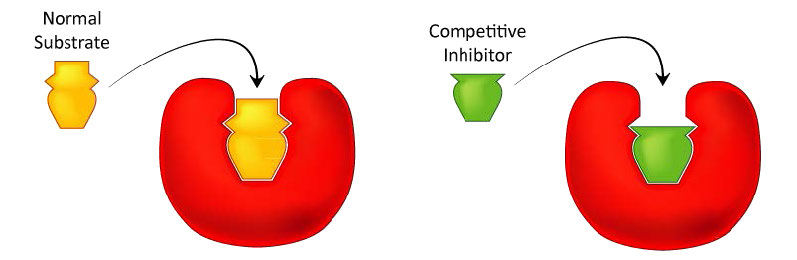
Figure 4.33 – Competitive inhibitors resemble the normal substrate and compete for binding at the active site. Image by Aleia Kim
Inhibitor binding
When the drug methotrexate is present, some of the DHFR enzyme binds to it, instead of to folate, and during the time methotrexate is bound, the enzyme is inactive and unable to bind folate. Thus, the enzyme is inhibited. Notably, the binding site on DHFR for methotrexate is the active site, the same place that folate would normally bind. As a result, methotrexate ‘competes’ with folate for binding to the enzyme. The more methotrexate there is, the more effectively it competes with folate for the enzyme’s active site. Conversely, the more folate there is, the less of an effect methotrexate has on the enzyme because folate outcompetes it.
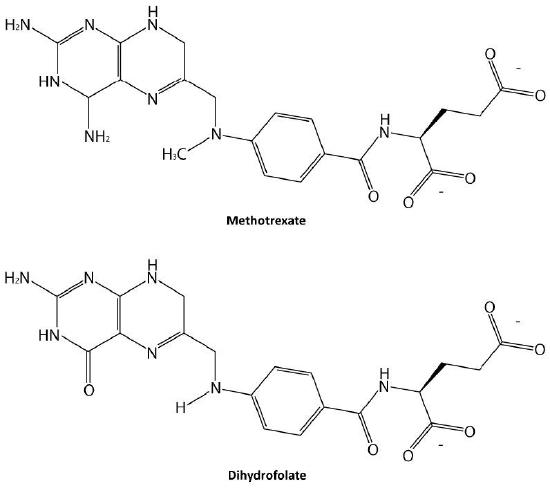
Figure 4.34 – Methotrexate and dihydrofolate. Image by Ben Carson
Non-competitive inhibition
A second type of inhibition employs inhibitors that do not resemble the substrate and bind not to the active site, but rather to a separate site on the enzyme (Figure 4.37). The effect of binding a non-competitive inhibitor is significantly different from binding a competitive inhibitor because there is no direct competition. In the case of competitive inhibition, the effect of the inhibitor can be reduced and eventually overwhelmed by flooding the enzyme with increasing amounts of substrate. With non-competitive inhibition, increasing the amount of substrate has no effect on the percentage of enzyme that is able to act. Indeed, in non-competitive inhibition, the percentage of enzyme inhibited remains the same through all ranges of substrate concentrations.
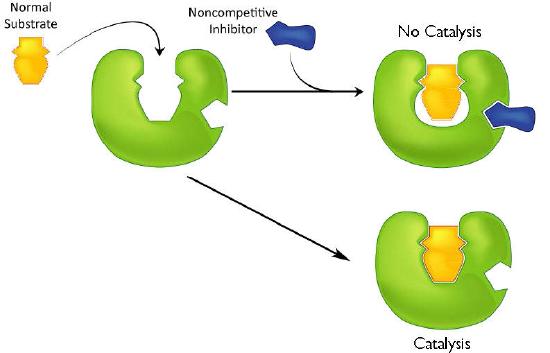
Figure 4.37 – Non-competitive inhibition – inhibitor does not resemble the substrate and binds to a site other than the active site. Image by Aleia Kim
Uncompetitive inhibition
A third type of enzymatic inhibition is that of uncompetitive inhibition. In this case the uncompetitive inhibitor (I) binds only to the enzyme-substrate (ES) complex (Figure 4.40). The inhibitor-bound complex forms mostly under concentrations of high substrate and the ES-I complex cannot release product while the inhibitor is bound.
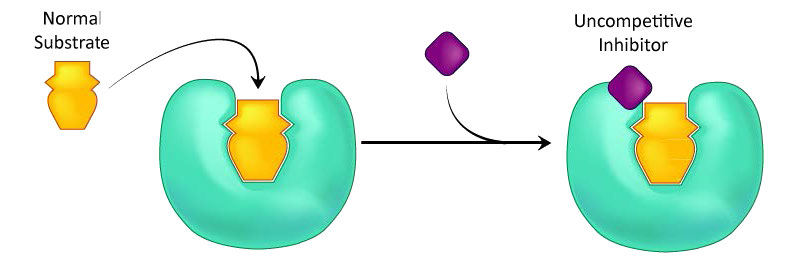
Figure 4.40 – Uncompetitive inhibition. Image by Aleia Kim
How can we tell what is happening between inhibitors and enzymes? Experimental evidence is obtained by measuring the rate of the enzyme-catalyzed reaction under various conditions. As substrate concentrations are increased, a competitive inhibitor will gradually ‘lose out’ to substrate and the rate of product formation will rise. But a similar effect will not be seen in the other forms of inhibition.
Additional experimental approaches are standard methods for such studies. These approaches have been enormously helpful to scientists studying natural systems as well as pharmaceutical interventions that target enzyme activity.
Suicide inhibition
In contrast to the first three types of inhibition, which involve reversible binding of the inhibitor to the enzyme, suicide inhibition is irreversible, because the inhibitor becomes covalently bound to the enzyme during the inhibition. Suicide inhibition rather closely resembles competitive inhibition because the inhibitor generally resembles the substrate and binds to the active site of the enzyme. The primary difference is that the suicide inhibitor is chemically reactive in the active site and makes a bond with it that precludes its removal. Such a mechanism is that employed by penicillin (Figure 4.43), which covalently links to the bacterial enzyme, DD transpeptidase and stops it from functioning. Since the normal function of the enzyme is to make a bond necessary for the peptidoglycan complex of the bacterial cell wall, the cell wall cannot properly form and bacteria cannot reproduce.
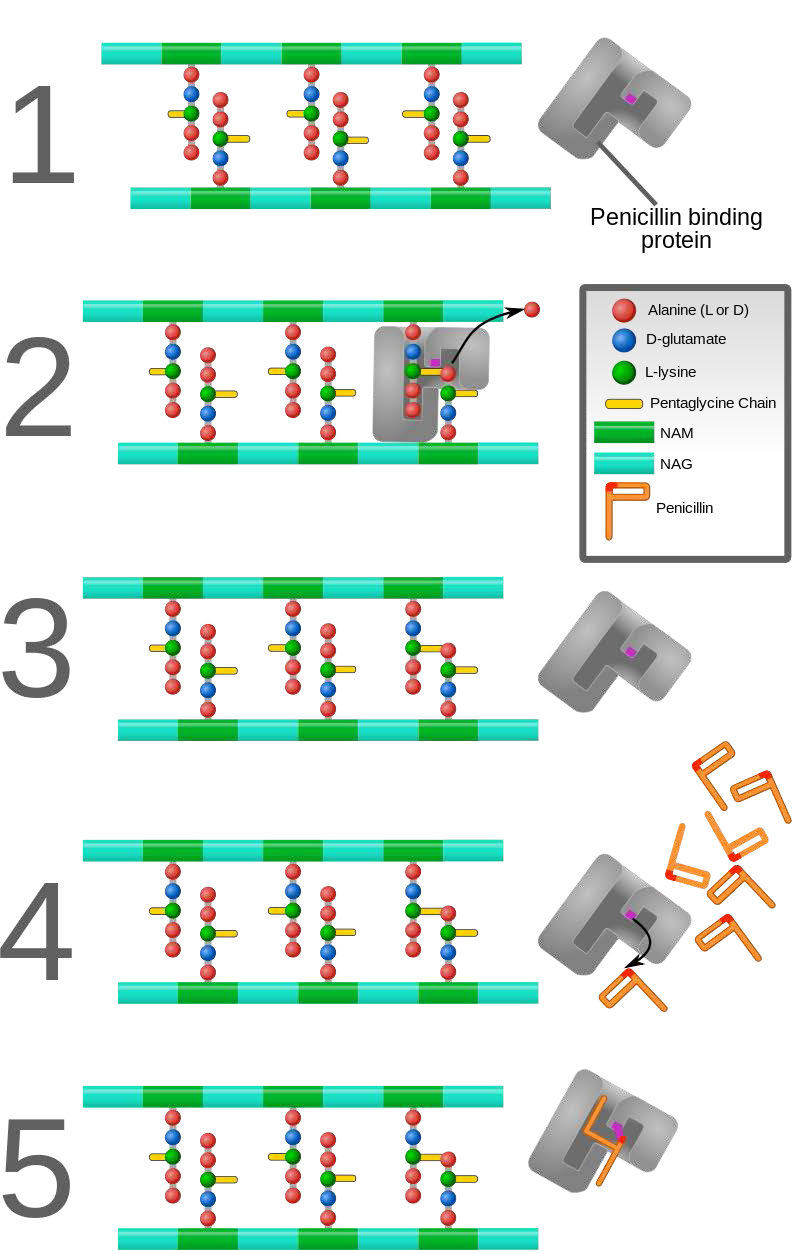
Figure 4.43 – Action of penicillin. DD-transpeptidase builds peptidoglycan layer of bacterial cell wall (1-3). Binding of penicillin by DD-transpeptidase stops peptidoglycan synthesis (4-5). Wikipedia
Control of enzymes
Enzymes are subject to controls from outside, but cells themselves also moderate and control enzyme activity. This allows an organism or cell to be responsive to its surroundings and physiological state.
There are four general ways this occurs:
- allosterism,
- covalent modification,
- access to substrate, and
- control of enzyme synthesis/breakdown.
Some enzymes are controlled by more than one of these methods.
Allosterism
The term allosterism refers to the fact that the activity of certain enzymes can be affected by the binding of small molecules at sites away from the active site.
When these effector molecules bind they cause the changes in the enzyme shape, as a result of binding.
Homotropic effectors, which are the same as the substrate molecules, usually are activators of the enzymes they bind to. from the less active T-state to the more active R-state, as the enzyme shifts its shape when a lot of substrate is around.
Away from the active site, enzymes can be either activated or inhibited when substances different than the substrate (heterotropic effectors) bind and induce different physical states (shapes, as it were) in an enzyme. These shifts alter the ability of the binding site to collect and hold substrate. On the other hand, an enzyme can be activated by effector binding as well.
Feedback inhibition
A special kind of allosteric control is exhibited by the enzyme HMG-CoA reductase, which catalyzes an important reaction in the pathway leading to the synthesis of cholesterol. Binding of cholesterol to the enzyme reduces the enzyme’s activity significantly. Cholesterol is not a substrate for the enzyme, so it is therefore a heterotropic effector.
Notably, though, cholesterol is the end-product of the pathway that HMG-CoA reductase catalyzes a reaction in. When enzymes are inhibited by an end-product of the pathway in which they participate, they are said to exhibit feedback inhibition.
Feedback inhibition always operates by allosterism and further, provides important and efficient control of an entire biochemical pathway. By inhibiting an early enzyme in a pathway, the flow of materials (and ATP hydrolysis required for their processing) for the entire pathway is stopped or reduced, assuming there are not alternate supply methods.
Pathway control
In the cholesterol biosynthesis pathway, stopping this one enzyme has the effect of shutting off (or at least slowing down) the entire pathway. This is significant because after catalysis by HMG-CoA reductase, there are over 20 further reactions necessary to make cholesterol, many of them requiring ATP energy. Shutting down one reactions stops all of them.
Covalent modification of enzymes
Some enzymes are synthesized in a completely inactive form and their activation requires covalent bonds in them to be cleaved. Such inactive forms of enzymes are called zymogens. Examples include the proteins involved in blood clotting and protein-destroying enzymes of the digestive system, such as trypsin, chymotrypsin, and pepsin. These enzymes can not be active where they are made (inside the pancreas) but only where they end up (inside the GI tract). When they become active in the tissues of the pancreas, pancreatitis occurs.
Cascades
For both the blood clotting enzymes and the digestive enzymes, the zymogens are activated in a protease cascade. This occurs when activation of one enzyme activates others in a sort of spreading chain reaction. In such a scheme the first enzyme activated proteolytically cleaves the second zymogen, causing it to be activated, which in turn activates a third and this may proceed through several levels of enzymatic action (Figure 4.50).
The advantage of cascades is that they allow a large amount of zymogens to become activated fairly quickly, since there is an amplification of the signal at each level of catalysis.
Blood clotting involves polymerization of a protein known as fibrin. Since random formation of fibrin is extremely hazardous because it can block the flow of blood, potentially causing heart attack/stroke, the body synthesizes fibrin as a zymogen (fibrinogen) and its activation results from a “cascade” of activations of proteases that arise when a signal is received from a wound. Similarly, the enzyme catalyzing removal of fibrin clots (plasmin) is also synthesized as a zymogen (plasminogen), since random clot removal would also be hazardous (see below also).
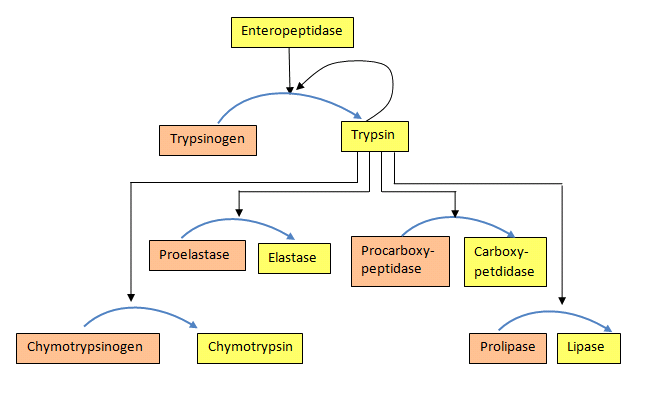
Figure 4.50 – Protease activation scheme. Wikipedia
Phosphorylation/dephosphorylation
Another common mechanism for control of enzyme activity by covalent modification is phosphorylation. The phosphorylation of enzymes (on the side chains of serine, threonine or tyrosine residues) is carried out by protein kinases. Enzymes activated by phosphorylation can be regulated by the addition of phosphate groups by kinases or their removal by phosphatases. Thus, this type of covalent modification is readily reversible, in contrast to proteolytic cleavage.
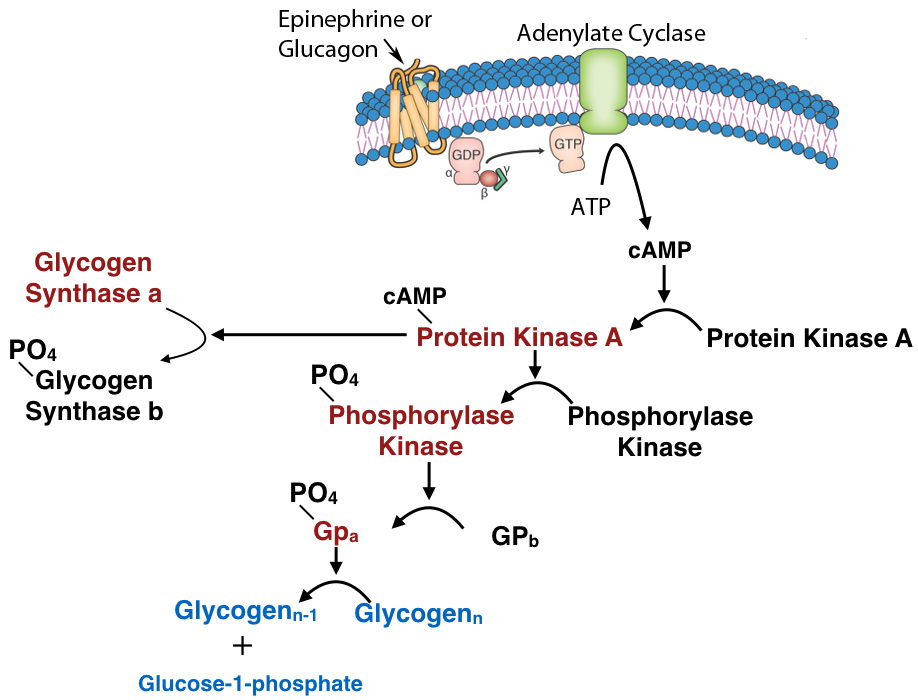
Figure 4.51 – Regulation by covalent modification of glycogen catabolism enzymes
Reduction/oxidation
An interesting covalent control of enzymes using reduction/oxidation is exhibited in photosynthetic plants. In the light phase of photosynthesis, electrons are excited by light and flow through carriers to NADP+, forming NADPH. Thus, in the light, the NADPH concentration is high. When NADPH concentration is high, the concentration of reduced ferredoxin (a molecule donating electrons to NADP+) is also high.
Reduced ferredoxin can transfer electrons to thioredoxin, reducing it. Reduced thioredoxin can, in turn, transfer electrons to proteins to reduce their disulfide bonds. Four enzymes related to the Calvin cycle can receive electrons from thioredoxin and become activated, as a result.
These include sedoheptulose 1,7-bisphosphatase, ribulose-5-phosphate kinase, fructose 1,6-bisphosphatase, and glyceraldehyde 3-phosphate dehydrogenase. Thus, in the light, electrons flow, causing NADPH to accumulate and ferredoxin to push electrons in the direction of these enzymes above, activating them and favoring the Calvin cycle. In the dark, the concentration of reduced NADPH, reduced ferredoxin, and reduced thioredoxin fall, resulting in loss of electrons by the Calvin cycle enzymes (oxidations that re-form disulfide bonds) and the Calvin cycle inactivates.
Other enzyme control mechanisms
Other means of controlling enzymes relate to access to substrate (substrate-level control) and control of enzyme synthesis from DNA. Hexokinase is an enzyme that is largely regulated by availability of its substrate, glucose. When glucose concentration is low, the product of the enzyme’s catalysis, glucose-6-phosphate, inhibits the enzyme’s function.
Regulation of enzymes by controlling their synthesis is covered later in the book in the discussion relating to control of gene expression.

