17 Acid-Fast and Endospore Staining
Emilie Miller, Ph.D
Acid-Fast Staining
Most bacterial species are either Gram positive or Gram negative, however some organisms have different cell wall properties that make them difficult to stain with this method. For example, some species of bacteria have a waxy lipid (mycolic acid) in their cell walls. These organisms generally do not Gram stain very well (those that do would usually appear gram positive) and are more clearly visible with the acid-fast staining technique.
Acid-fast staining was developed by Robert Koch in 1882 and later modified by other scientists. Koch used the method to observe the “tubercle bacillus”—what we now call Mycobacterium tuberculosis, in sputum samples. While acid-fast and gram staining are both differential stains, the acid-fast stain is much more specific. Many bacteria are either gram positive or gram negative, but very few are acid-fast. Two acid-fast genera that are important as human pathogens are Mycobacterium and Nocardia: Pathogenic species include M. tuberculosis, M leprae, M. bovis, M. avium, and N. asteroides. The protozoan parasite Cryptosporidium can also be stained using this procedure.
There are 2 different methods of acid-fast staining—both involve techniques that make the cell wall more permeable to the primary stain. The Ziehl-Neelson method uses steam heat to allow stain to penetrate, whereas the Kinyoun (cold method) uses a wetting agent mixed in with the primary stain. In this lab we will be using the Ziehl-Neelson method method.
Ziehl-Neelson Staining Procedure
- Prepare a slide with Mycobacterium smegmatis. (Alternatively, both bacteria may be mixed into one smear). Be sure to break up clumps of M. smegmatis before staining.
- Air dry and heat fix as usual.
- Add a small piece of bibulous paper on top of the sample
- Add carbol fuchsin (primary stain) on top of the paper to saturate: leave on for 5-7 minutes and place over a steam bath in the hood. Apply more carbol fuchsin as needed during the process
- Rinse with water. Note: not all of the primary stain will be removed by water in this step
- Decolorize with Acid-alcohol: 1-2 quick rinses
- Rinse with water.
- Add methylene blue (counterstain) and leave on for 2-3 minutes.
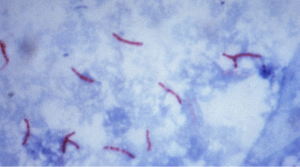 Rinse with water, blot dry, and view.
Rinse with water, blot dry, and view.
Acid-fast organisms retain the primary stain and will appear bright red: non acid-fast organisms are decolorized with acid-alcohol and pick up the methylene blue counterstain. Epithelial cells that may be present in a clinical sample will also appear blue.
Endospore staining
Endospores are the most resistant forms of life. They can resist desiccation (drying), boiling and radiation—in addition, disinfectants and antibiotics cannot penetrate an intact spore coat. For this reason they are difficult to eliminate from the environment with standard methods of disinfection, and they are difficult to treat in the case of an infection.
Endospores are a survival mechanism for the bacterial species that produce them. When conditions are favorable, vegetative bacterial cells will continue to grow and divide; however when nutrients are depleted, cells will begin to form endospores. Endospores are not metabolically active, but contain all the materials needed by cells to survive. When conditions for growth are again favorable, the spore will germinate and form a cell that is identical to the cell that produced it. Endospores are produced by certain types of Gram positive- bacilli, like Clostridium and Bacillus, as well as other species. Endospore-forming pathogens include C. tetani, C. botulinum, C. difficile, and B. anthracis.
Endospore Staining Procedure
- Prepare a smear with Bacillus subtilis on the slide.
- Air dry and heat fix as usual.
- Add a small piece of bibulous paper on top of the sample
- Add malachite green (stains spores) on top of the paper to saturate: leave on for at least 10 minutes. and place over a steam bath in the hood. Apply more as needed during the process
- Rinse briefly with water.
- Stain cells with safranin (stains vegetative cells) for 1 minute.
- Rinse with water, blot dry and view.
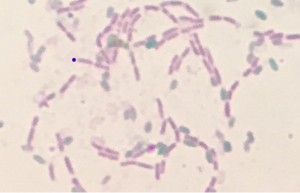
If the bacterial species is an endospore former, you will see pink vegetative cells as well as green oval-shaped endospores: non-spore formers will appear only as pink vegetative cells.

