21 Blood Agar
Emilie Miller, Ph.D
Hemolysis, the ability of an organism to lyse red blood cells and breakdown hemoglobin can be seen when sheep’s blood is added to the medium. Substances produced and released (exotoxins) which accomplish this are called hemolysins. The addition of blood has a two-fold purpose. It is an enrichment to support the growth of fastidious organisms and it is the substrate with which hemolysins, if present, will interact. In blood agar, the hemolytic activity can be visualized without an indicator substance usually added to most differential media.
Before inoculation, a blood agar plate appears red and opaque due to the large particulate red blood cells (RBCs) contained within. If an organism growing on the plate produces beta (β) hemolysins, the RBCs are completely destroyed or lysed and the medium surrounding the growth loses its opacity. If this is the case, the medium may become transparent enough that you may be able to read printing through the clearing.
If the blood cell membranes are partially lysed by the exotoxin, the RBC contents will leak out without the complete destruction of the blood cell. The hemoglobin from the RBC will be reduced to methemoglobin resulting in a green or brown discoloration to the medium surrounding the colony (Buxton, 2005). This incomplete hemolysis is called alpha (α) hemolysis. “On prolonged incubation, many alpha hemolytic organisms will begin to appear more clear, but if the surrounding medium contains any shades of brown or green the ‘hemolysis’ is still considered ‘alpha.’” (Buxton, 2005)
If the bacterium does not produce a hemolytic exotoxin, there will be no change to the RBCs, and the medium will remain opaque red. This lack of hemolysis is classified as gamma (ϒ) hemolysis.
Hemolysins are involved in the pathogenicity of bacteria. These membrane-disrupting exotoxins affect cell membrane function either by forming pores or by disrupting the phospholipid bilayer in host red blood cell membranes. They cause leakage of the cytoplasmic contents and cell lysis. They can affect other cells as well. The gram-positive bacterium Streptococcus pyogenes produces two types of streptolysins, O and S. Streptomycin O is not active in the presence of oxygen (oxygen labile), whereas streptolysin S is active in the presence of oxygen (oxygen stable). Other important pore- forming membrane-disrupting toxins include alpha toxin of Staphylococcus aureus and pneumolysin of Streptococcus pneumoniae. (OpenStax CNX, 2018)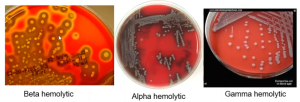
Blood Agar (TSB w/ 5%SB) Pancreatic digest of casein 14.5 g/L Peptic digest of soybean meal 5.0 g/L Sodium chloride 5.0 g/L, Agar 14.0 g/L, Defibrinated Sheep Blood 5.0%
Materials per student:
1 blood agar plate (TSA w/ 5% SB)
Cultures
Fresh overnight broth cultures of 4 microbes
Procedure Lab 1
- .Obtain one blood agar plate
- Add the following to the bottom of the plate around the edge: your name, date.
- On the bottom, divide each plate into 4 sections. Label each section with an abbreviation for each organism. Write small but legibly! Each plate will be inoculated with each of the 4 organisms.
- Aseptically make a short (1 cm) streak-stab inoculation of each organism in the corresponding section.
- After obtaining cells on the loop, begin the surface streak at point 1 and ending with a stab at point 2.
- Inoculate each organism into its area with a short straight line/spot inoculation followed by a stab at the end of the streak line.
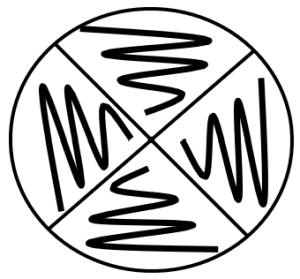
- Keep the inoculation lines short and away from other inoculations on the plate.
- Be sure to make each inoculation separately and refrain from “double dipping.”
- Be sure to hold the lid of the plate above the plate surface to protect it from airborne contaminants.
- It may help to set the plate on a piece of white scratch paper so that you can see the sector lines.
- Place the plates upside-down in the location designated for cultures to be incubated.
- They will be incubated for 24-48 hours at 37oC.
Procedure Lab 2
- Obtain your plates and make observations in the data table.
- Use “+” for growth and “–“for no growth. If growth is poor, simply write “poor growth” in the table.
- In the appearance column, describe any change in the surrounding medium or the growth itself. Hold the plate up to the light. You may see slight lighting of the medium or a complete clearing. The medium or the growth itself may appear green. Careful not to confuse a greenish appearance with a shadow created by the growth. If there is no change in appearance to the medium, write “no change.”
- Be sure to use the organism’s full scientific name written correctly.
- After making observations, dispose of plates in the hazardous waste.
References:
OpenStax CNX. (2018, Mar 19). OpenStax Microbiology. Retrieved from http://cnx.org/contents/e42bd376-624b-4c0f-972f-e0c57998e765@4.24

