31 Antibiotic Susceptibility Testing
Emilie Miller, Ph.D
The Kirby-Bauer disc diffusion test has long been used as a starting point for determining the susceptibility of specific microbes to various antimicrobial drugs. It can also be used to determine an antibiotic’s range of effectiveness.
The Kirby-Bauer assay starts with an agar plate on which a confluent lawn is inoculated with a patient’s isolated bacterial pathogen. Filter paper discs impregnated with known amounts of antibacterial drugs are placed on the agar surface. The antibiotic diffuses from the disc into the agar creating a concentration gradient. The drug’s concentration is highest near the disc and gets more dilute the greater the distance from the disc. The microbial cells interact with the drug at these varying concentrations. The minimum concentration that will keep the bacterial cells from growing is called the minimum inhibitory concentration, MIC. At concentrations above the MIC, the organism will not grow. After incubation, this antibacterial activity is observed as a clear circular zone of inhibition around the drug-impregnated disc. The diameter of the zone of inhibition, measured in millimeters and compared to a standardized chart, determines the susceptibility or resistance of the bacterial pathogen to the drug.
There are multiple factors that determine the size of a zone of inhibition in this assay, including drug solubility, rate of drug diffusion through agar, the thickness of the agar medium, and the drug concentration impregnated into the disc and the MIC.
 Mechanisms of action of various antibiotics can be found at this link or QRS code. (OpenStax CNX, 2018)
Mechanisms of action of various antibiotics can be found at this link or QRS code. (OpenStax CNX, 2018)
Materials per student pair
Cell Spreader
2 Agar plates
Antibiotic Discs
Forceps
Procedure lab 1
- Assemble all materials. Label each plate with all 4 components, one organism per plate.
- It is not necessary to add the name of each antibiotic to the label. Each disc has a code that denotes the antibiotic and its concentration.
- With a pen, divide the plates in 6 parts.
- Inoculate each plate with a different culture using the cotton swab to obtain a uniform bacterial lawn as follows.
- Determine which end of the swab package contains the cotton bulb. Open the package at opposite end exposing only a short length of the stick. Remove one of the swabs.
- Aseptically insert the swab into the diluted culture.
- As the swab is removed, press the bulb against the side of the culture tube to press out excess liquid. Aseptically replace the test tube cap and set the tube in the rack.
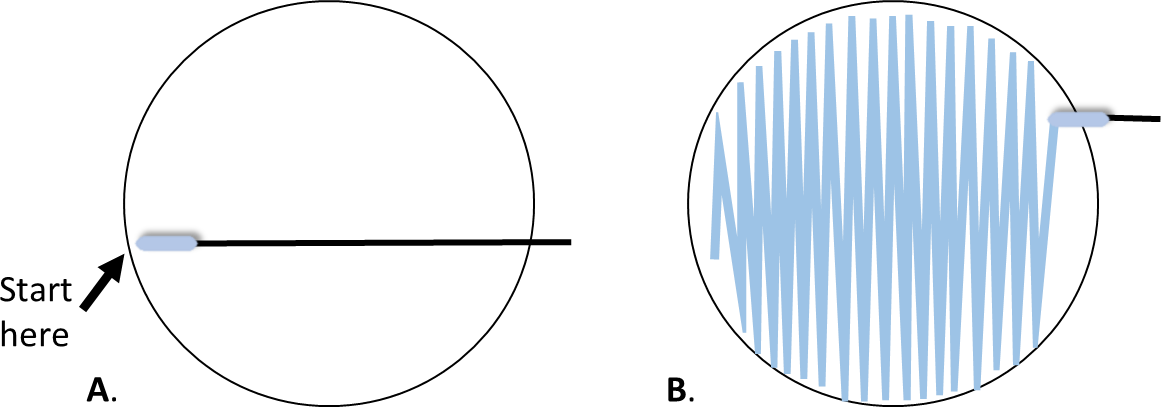
- With the plate right side up, hold the lid above the plate as a shield. Starting at the point farthest away from your dominant hand (figure 1A), swab the agar surface streaking back and forth from one side of the plate to the other. Attempt to cover the entire surface of the agar without space between the streaks.
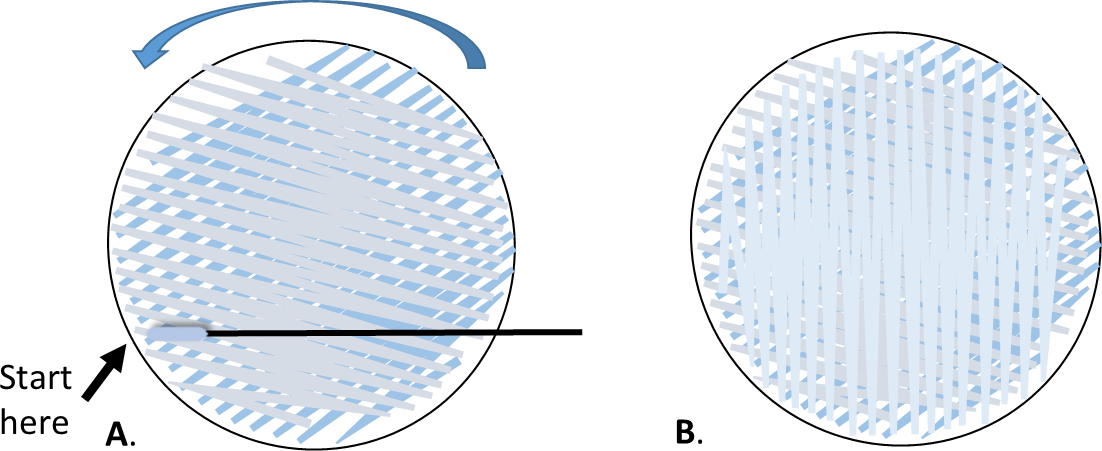
- Rotate the plate approximately 60o, and repeat with the same swab.

- Rotate 60o again, swab one more time.
- Dispose of the swab in the biohazard waste.
- After you have inoculated each plate with the respective organism, place one disc of each antibiotic on each plate in each of the 6 parts. Use identical antibiotics for each plate.
- Place one plate right side up on a white piece of scratch paper so that you can see the placement dots.
- Place the plates up-side-down in the location designated for cultures to be incubated.
- They will be incubated for 24 hours at 37oC.
Procedure lab 2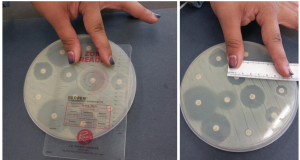
- Observe your plates by measuring and recording the diameters of each zone of inhibition in millimeters.
- Compare each diameter to the interpretive chart and determine each organism’s susceptibility to each antibiotic.
Zone Diameter Interpretive Chart
Adapted from (BD BBL Sensi-Disc Antimicrobial Susceptibility Test Discs package insert)
|
|
|
|
Zone Diameter Interpretive Standards (mm) |
||
|
Antimicrobial agent |
code |
Disc Potency |
Resistant |
Intermediate |
Susceptible |
|
Chloramphenicol Enterobacteriaceae P. aeruginosa, Staphylococci, Enterococci |
C-30 |
30 µg |
≤12 |
13-17 |
≥18 |
|
Ciprofloxacin Enterobacteriaceae P. aeruginosa, Staphylococci, Enterococci |
CIP-5 |
5 µg |
≤15 |
16-20 |
≥21 |
|
Penicillin Staphylococci |
P-10 |
10 µg |
≤28 |
|
≥29 |
|
Penicillin Enterococci |
P-10 |
10 µg |
≤14 |
|
≥15 |
|
Streptomycin Enterobacteriaceae |
S-10 |
10 µg |
≤11 |
12-14 |
≥15 |
|
Tetracycline Enterobacteriaceae P. aeruginosa, Staphylococci, Enterococci |
Te-30 |
30 µg |
≤14 |
15-18 |
≥19 |
|
Trimethoprim Enterobacteriaceae Staphylococci |
TMP-5 |
5 µg |
≤12 |
13-17 |
≥18 |
References:
BD BBL Sensi-Disc Antimicrobial Susceptibility Test Discs package insert. (n.d.). Zone Diameter Interpretive Chart.
OpenStax Microbiology, Microbiology . OpenStax CNX. Mar 19, 2018 http://cnx.org/contents/e42bd376- 624b-4c0f-972f-e0c57998e765@4.24. (n.d.).