15 Streaking for Isolation
Emilie Miller, Ph.D
If a single bacterial cell is placed on the surface of a TSA agar plate and allowed to multiply for 24 to 48 hours, it would grow into a mass of cells visible to the human eye called a colony. Colonies formed by the same microbial species growing on the same medium will all look alike. This is because the cell shape, pigmentation, division plane, rate of cell division and other characteristics of the organism result in the progeny cells stacking on one another in a pattern resulting in a characteristic colony form.
 If you swab a door handle, where bacteria are likely to be present, and then pass the swab across the surface of a TSA plate, cells would be deposited onto the surface from the swab. Initially, the swab may have a fairly high concentration of cells and the area touched by it will have lots of different cell types placed close together. After these grow up, the cells’ progeny will crowd together and overlap with other cells’ progeny forming areas called confluent growth. This is the type of growth you observed on the surface of the TSA slants in the Basic Aseptic Transfers exercise.
If you swab a door handle, where bacteria are likely to be present, and then pass the swab across the surface of a TSA plate, cells would be deposited onto the surface from the swab. Initially, the swab may have a fairly high concentration of cells and the area touched by it will have lots of different cell types placed close together. After these grow up, the cells’ progeny will crowd together and overlap with other cells’ progeny forming areas called confluent growth. This is the type of growth you observed on the surface of the TSA slants in the Basic Aseptic Transfers exercise.
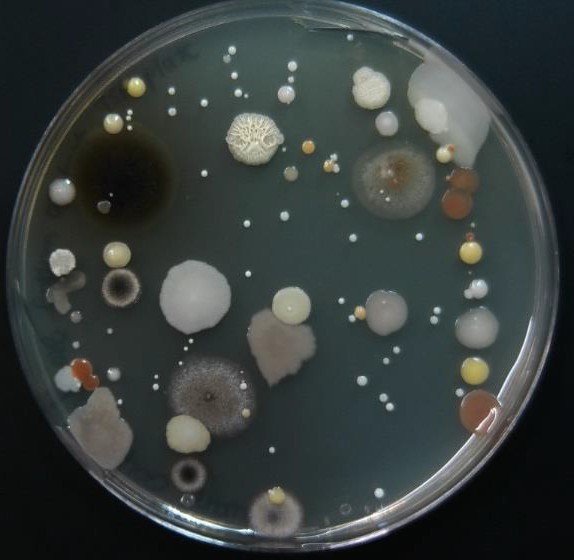
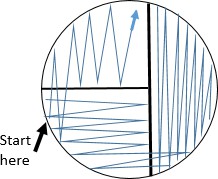
As the swab moves across the agar leaving cells on the agar in a zig zag pattern, regions touched later in the process will have fewer and fewer cells. Individual cells are far enough apart that each one would grow into a discrete isolated colony. The result may look something like the figure to the right, Because the door handle likely has a variety of microbes on it, there are numerous colony forms. This technique is called a zig zag streak and is one type of isolation streak method.
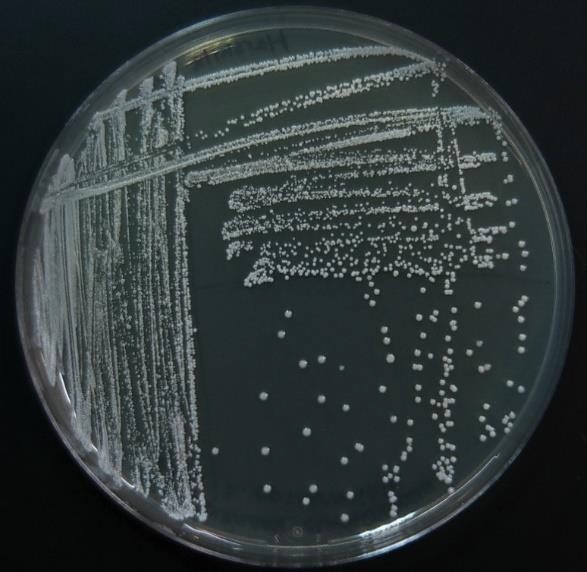
Now consider streaking a sample from a pure TSA broth culture prepared for you. If there is visible turbidity, there will be a high density of cells. If you used the same zig zag streaking pattern, the cells would never be reduced in concentration such that you could get isolated colonies. In order to reduce the cell density on the surface of the plate, we can use a T-Streak or a quadrant streak technique.
The T-streak and quadrant streak are forms of dilutions on a solid surface (Franklund, 2018). In these two techniques the plate is divided into sections. Bacteria are deposited in the first section at full strength from the source. Then the inoculating loop is sterilized. From this point on, no additional cells are added to the agar surface. The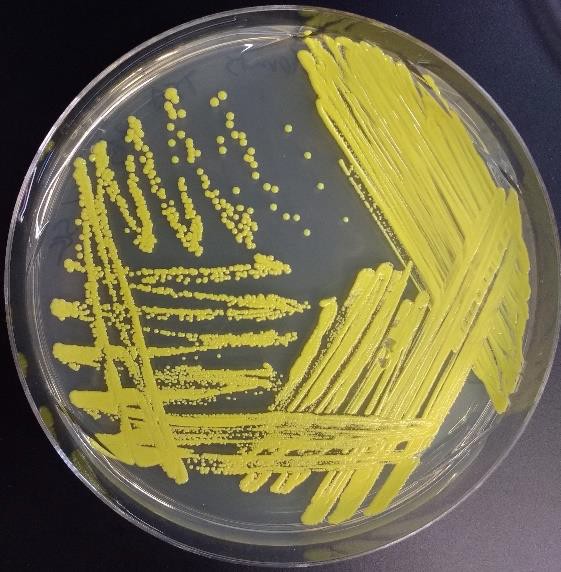 sterile loop is used to spread out cells that have already been placed on the plate. After spreading cells from the first area to the second, the loop is sterilized again. This eliminates extra cells from the loop. The sterile loop is used to spread some cells from the second area into the third area diluting them further. (In a quadrant streak, cells are spread into a forth area as described.) After all the regions have been inoculated, the hope is that in the last section cells are far enough apart so that they grow up into isolated colonies.
sterile loop is used to spread out cells that have already been placed on the plate. After spreading cells from the first area to the second, the loop is sterilized again. This eliminates extra cells from the loop. The sterile loop is used to spread some cells from the second area into the third area diluting them further. (In a quadrant streak, cells are spread into a forth area as described.) After all the regions have been inoculated, the hope is that in the last section cells are far enough apart so that they grow up into isolated colonies.
This technique allows one to observe isolated colonies and characterize them and determine if your observations are consistent with our expectations for the organism you are working with. If you are working with a pure culture, you would expect that all the colonies would look the same, similar size, color, shape etc. One or more different looking colonies indicates your culture was contaminated or you created contamination by poor aseptic technique.
Zig Zag Streak
- Aseptically obtain a sample from the media using a sterile inoculating loop.
- Turn the agar plate right-side-up.
- Hold the plate lid so that it acts as a shield protecting the agar surface from microbes falling from the air.
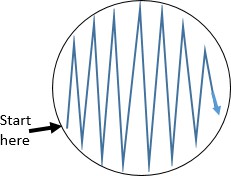 Starting the streak on the side of the plate farthest from your dominant hand, pass the loop on the surface of the agar in a zig zag pattern filling the surface of the plate. See figure to the right.
Starting the streak on the side of the plate farthest from your dominant hand, pass the loop on the surface of the agar in a zig zag pattern filling the surface of the plate. See figure to the right.- Replace the lid, and immediately flame the loop.
- Place the plate upside-down for incubation.
- Some tips for a good zigzag streak: Use as much of the agar surface as you can. Make broad strokes that span the width of the plate.
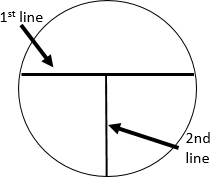 T-streak
T-streak
The steps of the T-streak are as follows.
- Draw lines on the bottom of your plate as shown in figure to the right. The first line should be a little off center. Drawing these is not absolutely necessary, but they help when you are first learning.
- Aseptically obtain a loopful of the culture and set the tube back in a rack.
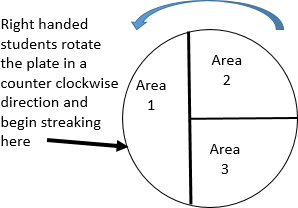 Turn the plate right-side up and place it on a piece of white scratch paper so that the lines can be seen.
Turn the plate right-side up and place it on a piece of white scratch paper so that the lines can be seen.- Rotate the plate so that area 1 is farthest from your dominate hand.
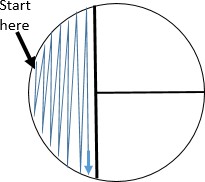 Holding the plate lid so that it acts as a shield protecting the agar surface from microbes falling from the air, pass the loop on the surface of the agar in area 1 in the pattern shown.
Holding the plate lid so that it acts as a shield protecting the agar surface from microbes falling from the air, pass the loop on the surface of the agar in area 1 in the pattern shown.- Replace the plate lid, flame the loop, and allow it to cool.
- Rotate the plate 90o.
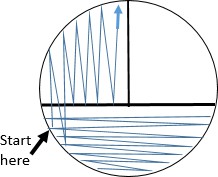 WITHOUT OBTAINING ADDITIONAL CULTURE, spread the cells already placed on the agar surface with the sterile loop by streaking in the pattern shown. This step pulls highly concentrated cells from area 1 into area 2 spreading them out.
WITHOUT OBTAINING ADDITIONAL CULTURE, spread the cells already placed on the agar surface with the sterile loop by streaking in the pattern shown. This step pulls highly concentrated cells from area 1 into area 2 spreading them out.
- Replace the plate lid, INCINERATE THE LOOP, and allow it to cool. Rotate the plate 90o.
 Again, WITHOUT OBTAINING ADDITIONAL CULTURE, spread some of the cells from area 2 into area 3 by streaking in the pattern shown.
Again, WITHOUT OBTAINING ADDITIONAL CULTURE, spread some of the cells from area 2 into area 3 by streaking in the pattern shown.
- Replace the plate lid and flame the loop again before setting it down. Turn the plate upside-down for incubation and storage.
In this technique it is essential that you flame your loop after each area is inoculated. This is what reduces the cell density because you are spreading cells already on the plate. You are not adding additional cells by using a loop with cells on it. You eliminate additional cells by the flame step. After incubation, the goal is at least 3 well isolated colonies.
Keep in mind that you want to use as much of the agar surface as possible. Your streaks should span the width of the plate. The loop passes through the previous area 2- 3 times and then does not touch that area again. If you keep touching the previous high density streak, you will pull too many cells into the next area and will not reduce the number enough to get isolated colonies. If you do not cross over the previous area enough, you will not have enough cells in the next one.
Each of the streaks is at a 90o angle to the previous streak and parallel to the lines you drew. The areas have similar areas. If you choose to cool your loop in the agar, always use a spot close to the edge and away from any previous streak. The resulting growth pattern should be confluent in area 1, more diffuse in area 2 and least growth in area 3.
Materials per student/group
2 TSA plates, inoculation loop, Bunsen burner, striker, pen
Pure cultures of microbe in broth
Practice: https://learn.chm.msu.edu/vibl/content/streakplate.html
Procedure lab 1
 Draw the Zig Zag streak pattern and the T-streak pattern in the circles on the observations page.
Draw the Zig Zag streak pattern and the T-streak pattern in the circles on the observations page.- Label one plate with your name, date, microorganism, and medium. Keep the writing on the edge of the plate so that it will not obscure the growth on the plate.
- On this plate, you will perform a zig zag inoculation from prepared culture.
- Follow the steps for making a Zig zag inoculation.
 You will now make a T-Streak of the pure culture.
You will now make a T-Streak of the pure culture. - Label the bottom of the plate with the 4 components. Include the specific microbe that you are using. (Do not use “pure culture.”)
- Follow the procedure for a T-Streak.
- Take all of your plates to the location to be incubated. Be sure to place them upside-down. They will be incubated 25-37oC for 24-48 hours.
Procedure lab 2
- Observe your 2 plates. Fill in the observations table. For the source, write in the full scientific name of the organism.
- Evaluate your technique using the criteria listed. Get input from another lab member and instructor if needed. Check the items that characterize your plate.
- Dispose of your plates in the biohazard waste.
References:
Franklund, C. (n.d.). Microbiology Laboratory Manual, Observing and Recording the Microbial World. Farris State University. Retrieved 2018, from https://github.com/WeeBeasties/microbiology- laboratory-manual

