23 SIM Medium
Emilie Miller, Ph.D
SIM is an example of a combination medium, meaning that one can determine several bacterial activities/characteristics through the use of one medium. SIM medium tests for sulfur reduction, indole production and motility. The form of medium used for this test is an agar deep. SIM Medium contains the following: pancreatic digest of casein, peptic digest of animal tissue, ferrous ammonium sulfate Fe(NH4)2(SO4), sodium thiosulfate Na2S2O3, agar (3.5 g/L) and distilled water.
Sulfur can be reduced producing hydrogen sulfide by bacteria in two unrelated ways. One process occurs during putrefaction. When proteins putrefy, the resulting foul “rotten egg” smell is due to the production of hydrogen sulfide gas, H2S. Hydrogen sulfide is a byproduct of the conversion of the amino acid cysteine to pyruvate by the enzyme cysteine desulfurase. The second mode of H2S generation involves anaerobic respiration. In some prokaryotes, thiosulfate (S2O32-) is the terminal electron acceptor in an anaerobic ETS. When thiosulfate is reduced (picks up electrons) the result is H2S gas. In either case, invisible H2S gas is produced.
Because hydrogen sulfide gas is colorless (though not odorless!) SIM medium uses an indicator reaction. Iron (supplied by ferrous ammonium sulfate) in the medium combines with H2S gas to form iron sulfide, FeS, a black precipitate. Any black color in the medium is a positive test for sulfur reduction. Unfortunately, this test does not distinguish between the hydrogen sulfide produced as a result of putrefaction and hydrogen sulfide produced at the end of an anaerobic ETS.
Indole is produced during the conversion of tryptophan, an amino acid, to pyruvate and ammonia by the enzyme tryptophanase. Indole production indicates tryptophanase activity. Kovac’s reagent, added after incubation, will turn pink when it combines with indole.
Motility is the ability of a microbe to “swim” using flagella. The reduced agar content of this medium, 3.5 g/L compared to 12-15 g/L in most solid media, creates a semi liquid environment allowing motile cells to spread from their original placement. The stab technique (see the description below) deposits cells in a straight line down the center of the deep. If growth is observed beyond the stab line into the periphery of the tube, the test is positive for motility. Avoid confusing growth produced by the lateral movement of the needle during an imperfect stab inoculation with actual motility. Rotating the tube for a side view and comparing each experimental tube to the uninoculated tube will help you determine if growth is confined to the original inoculation line, or has truly spread into the periphery of the tube.
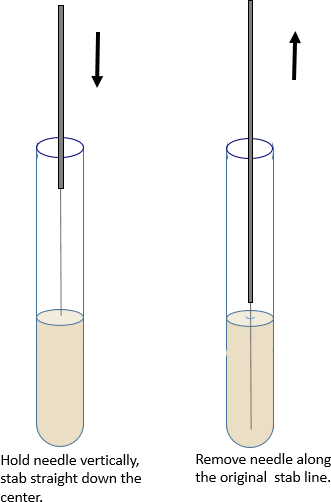
Stab Inoculation
- Aseptically obtain cells on the end of the inoculating needle
- Holding the deep tube in your non-dominant hand, remove the cap and flame the mouth of the tube as you normally do with a tube.
- Holding the needle vertically, stab the agar straight down the center to within a quarter inch of the bottom. Then draw it straight back out of the tube. Try to follow the original stab line when removing the needle.
- Again, heat the mouth of the tube after withdrawing the transfer instrument. Replace the cap loosely and set the tube in the test tube rack.
- Immediately flame the inoculating needle for a full 10 seconds before setting it down.
Materials per student pair
2 SIM deep tubes
Inoculating needle
Lab 2: Indole reagent (Kovac’s)
Cultures
Serratia marcescens
E. coli
Procedure Lab 1
- Label your tubes with each of the organisms. Don’t forget to include the other components of the label.
- Aseptically obtain an inoculum from each culture.
- Aseptically stab inoculate the corresponding tubes.
- Place the tubes for incubation for 24-48 hours at 37oC.
Procedure Lab 2
- Make observations for sulfur reduction and motility first. As in the other DT exercises, observations are what you see, result is “+” or “-“and interpretation refers to the result’s meaning.
- In the sulfur reduction data table, observe the location of any black color.
- For motility, it helps to compare the experimental tubes with the sham inoculated tube. Hold a paper with small print behind the tubes. Try to read the printing through the tubes. By comparing the inoculated tubes with an uninoculated tube you can determine if there is radiating (fuzzy) growth from the stab line. Be sure that you can distinguish between non-motile growth confided to the stab line (in 2 dimensions) and actual radiating growth, 360o around the inoculation.
- After you have observed motility and sulfur reduction, you can add the reagent for the indole test. The Kovac’s reagent is kept in the hood.
- Place 3-4 drops of the reagent on the agar surface. Replace the test tube cap. If indole is present, you will see a pink color develop within 2-3 minutes.
- When finished, place your experimental tubes in the biohazard waste.