11 Scientific Notation

Powers of Ten
Decimal notation is based on powers of 10: 0.1 is ![]() , 0.01 is
, 0.01 is ![]() , 0.001 is
, 0.001 is ![]() , and so on.
, and so on.
We represent these powers with negative exponents: ![]() ,
, ![]() ,
, ![]() , etc.
, etc.
Note: This is true for any base, not just 10, but we will focus only on 10 in this course.
With our base 10 number system, any power of 10 can be written as a 1 in a certain decimal place.
If you haven’t watched the video “Powers of Ten” from 1977 on YouTube, take ten minutes right now and check it out. Your mind will never be the same again.
Scientific Notation
Let’s consider how we could rewrite some different numbers using these powers of 10.
Let’s take 50,000 as an example. 50,000 is equal to ![]() or
or ![]() .
.
Looking in the other direction, a decimal such as 0.0007 is equal to ![]() or
or ![]() .
.
The idea behind scientific notation is that we can represent very large or very small numbers in a more compact format: a number between ![]() and
and ![]() , multiplied by a power of
, multiplied by a power of ![]() .
.
Note: An integer is a number with no fraction or decimal part: … -3, -2, -1, 0, 1, 2, 3 …
Although we generally try to avoid using the “x” shaped multiplication symbol, it is frequently used with scientific notation.
Exercises
- Earth and Mars are two of the smaller planets. Earth has a mass of approximately 5,970,000,000,000,000,000,000,000 kilograms, and Mars has a mass of approximately 639,000,000,000,000,000,000,000 kilograms. Can you determine which mass is larger?
Clearly, it is difficult to keep track of all those zeros. Let’s rewrite those huge numbers using scientific notation.
Exercises
- Earth has a mass of approximately
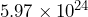 kilograms, and Mars has a mass of approximately
kilograms, and Mars has a mass of approximately 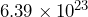 kilograms. Can you determine which mass is larger?
kilograms. Can you determine which mass is larger?
It is much easier to compare the powers of 10 and determine that the mass of the Earth is larger because it has a larger power of 10. You may be familiar with the term order of magnitude; this simply refers to the difference in the powers of ![]() of the two numbers. Earth’s mass is one order of magnitude larger because 24 is 1 more than 23.
of the two numbers. Earth’s mass is one order of magnitude larger because 24 is 1 more than 23.
Exercises
Suppose someone tells you their salary is “six figures”.
- To the nearest dollar, what is their minimum possible salary? Write the answer in standard notation and in scientific notation.
- To the nearest dollar, what is their maximum possible salary? Write the answer in standard notation and in scientific notation.
We can apply scientific notation to small decimals as well.
Exercises
- The radius of a hydrogen atom is approximately 0.000000000053 meters. The radius of a chlorine atom is approximately 0.00000000018 meters. Can you determine which radius is larger?
Again, keeping track of all those zeros is a chore. Let’s rewrite those decimal numbers using scientific notation.
Exercises
- The radius of a hydrogen atom is approximately
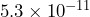 meters. The radius of a chlorine atom is approximately
meters. The radius of a chlorine atom is approximately 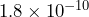 meters. Can you determine which radius is larger?
meters. Can you determine which radius is larger?
The radius of the chlorine atom is larger because it has a larger power of 10; the digits 1 and 8 for chlorine begin in the tenth decimal place, but the digits 5 and 3 for hydrogen begin in the eleventh decimal place.
Scientific notation is very helpful for really large numbers, like the mass of a planet, or really small numbers, like the radius of an atom. It allows us to do calculations or compare numbers without going cross-eyed counting all those zeros.
Exercises
Write each of the following numbers in scientific notation.
Convert the following numbers from scientific notation to standard decimal notation.
Multiplying & Dividing with Scientific Notation
You may be familiar with a shortcut for multiplying numbers with zeros on the end; for example, to multiply ![]() , we can multiply the significant digits
, we can multiply the significant digits ![]() and count up the total number of zeros, which is five, and write five zeros on the back end of the
and count up the total number of zeros, which is five, and write five zeros on the back end of the ![]() :
: ![]() . This shortcut can be applied to numbers in scientific notation.
. This shortcut can be applied to numbers in scientific notation.
Exercises
Multiply each of the following and write the answer in scientific notation.
When the numbers get messy, it’s probably a good idea to use a calculator. If you are dividing numbers in scientific notation with a calculator, you may need to use parentheses carefully. The following rule is true, but you may just want to use a calculator instead.
Exercises
- New Jersey has the highest population density of the 50 states.[1] Its population is
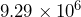 people and its land area is
people and its land area is 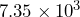 square miles. Divide these numbers to find the population density in people per square mile.
square miles. Divide these numbers to find the population density in people per square mile. - California has the highest population density of all states west of the Mississippi River.[2] Its population is
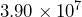 people and its land area is
people and its land area is 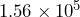 square miles. Divide these numbers to find the population density in people per square mile.
square miles. Divide these numbers to find the population density in people per square mile. - The mass of a proton is
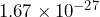 kg. The mass of an electron is
kg. The mass of an electron is 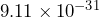 kg. Divide these numbers to determine approximately how many times greater the mass of a proton is than the mass of an electron.
kg. Divide these numbers to determine approximately how many times greater the mass of a proton is than the mass of an electron.
Engineering Notation
Closely related to scientific notation is engineering notation, which uses powers of ![]() . This is the way large numbers are often reported in the news; if roughly
. This is the way large numbers are often reported in the news; if roughly ![]() people live in Oregon City, we say “thirty-seven thousand” and we might see it written as “37 thousand”; it would be unusual to think of it as
people live in Oregon City, we say “thirty-seven thousand” and we might see it written as “37 thousand”; it would be unusual to think of it as ![]() and report the number as “three point seven ten thousands”.
and report the number as “three point seven ten thousands”.
One thousand = ![]() , one million =
, one million = ![]() , one billion =
, one billion = ![]() , one trillion =
, one trillion = ![]() , and so on.
, and so on.
In engineering notation, the power of ![]() is always a multiple of
is always a multiple of ![]() , and the other part of the number must be between
, and the other part of the number must be between ![]() and
and ![]() .
.
A number is written in engineering notation if it is written in the form ![]() , where
, where ![]() is a multiple of
is a multiple of ![]() and
and ![]() is any real number such that
is any real number such that ![]() .
.
Note: Prefixes for large numbers such as kilo, mega, giga, and tera are essentially engineering notation, as are prefixes for small numbers such as micro, nano, and pico. We’ll see these in Module 16.
Exercises
Write each number in engineering notation, then in scientific notation.
- The U.S. population is around
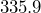 million people.[3]
million people.[3] - The world population is around
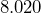 billion people.[4]
billion people.[4] - The U.S. national debt is around
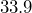 trillion dollars.[5]
trillion dollars.[5]
- Divide the U.S. national debt by the U.S. population to determine the amount of debt per person.
For a visualization of the relative sizes of a million, a billion, and a trillion, see this graphic made by Chris Kane, graphic artist and my friend from high school (PDF file).
- Source: https://en.wikipedia.org/wiki/New_Jersey ↵
- Source: https://en.wikipedia.org/wiki/List_of_states_and_territories_of_the_United_States_by_population_density ↵
- Retrieved from https://www.census.gov/popclock/, January 1, 2024 ↵
- Retrieved from https://www.census.gov/popclock/, January 1, 2024 ↵
- Retrieved from https://fiscaldata.treasury.gov/datasets/debt-to-the-penny/, January 1, 2024 ↵

