90 Preventing Hyperthermia

Hyperthermia, as opposed to hypothermia, occurs when body temperature increases as thermal energy builds up the body because heat is not transferred out of the body fast enough to keep up with the body’s thermal power. We can try to avoid such a situation by minimizing our work output to reduce overall thermal power (remember, the body has low efficiency so doing work means generating thermal energy). We can also use our understanding of the conduction, convection, and thermal radiation to ensure maximum heat transfer away from the body. For example, we can minimize the thickness of clothing to increase conduction, wear light colored clothing to reduce radiation absorbed from the sun, and encourage air circulation (convection).

Sweating
In some cases our thermal power outpaces the rate at which we exhaust heat by conduction, convection and radiation. Our strategy to deal with this situation is sweating. When we sweat some of the water on our skin evaporates into a water vapor. Only the molecules with the most kinetic energy are able to escape the attraction of their fellow water molecules and enter the air. Therefore the evaporating molecules remove more than a fair share of the thermal energy (thermal energy is just molecular kinetic energy remember). The remaining liquid water molecules then have less thermal energy on average, so they are at a lower temperature and must absorb more energy from your body as they come to thermal equilibrium with your body again. This evaporation process allows the body to dump thermal energy even when the environment is too warm for significant heat loss by conduction, convection, and radiation. The amount of energy removed by evaporation is quantified by the latent heat of vaporization (Lv). For water Lv = 2,260 kJ/kg, which means that for every kilogram of sweat evaporated, 2260 kiloJoules of energy is transferred away from the skin.
Everyday Example
A person working in an environment that happens to be very close to body temperature (about 100 °F) would not be able to get rid of thermal energy by conduction, convection, or radiation. If the person was working hard and generating about 250 W of thermal power (similar to the thermal power while shivering) then how much sweat would need to be evaporated each hour to keep their body temperature from rising?
In order to keep the body temperature from rising the person needs to get rid of 250 W of thermal energy, that’s 250 J/s. Let’s convert that to Joules per hour:
(1) ![]()
Each kilogram of water that evaporates removes 2,260,000 J of energy, so only a fraction of a kilogram will need to be evaporated every hour:
(2) ![]()
The body would need to evaporate 0.4 kg per hour. Water has a density of about 1 kg/L, so that would be a volume of 0.4 L/hr, or roughly 1.7 cups/hr, or 13.5 fluid oz/hr.
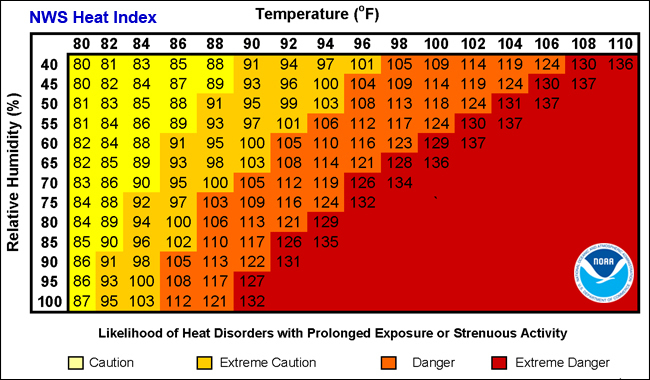
Heat Index
The rate at which water will evaporate depends on the liquid temperature and the relative humidity of the surrounding air. The relative humidity compares how many water molecules are in the vapor phase relative to the maximum number that could possibly be in the vapor phase at the current temperature. A relative humidity of 100% means that no more water molecules can be added to the vapor phase. If the humidity is high, then evaporation will be slow and may not provide sufficient cooling. The heat index takes into account both air temperature and the relative humidity to determine how difficult it will be for your body to exhaust heat. Specifically, the heat index provides the theoretical air temperature that would be required at 20% humidity to create the same difficulty in exhausting heat as the actual temperature and humidity. Heat index values were devised for shady conditions with a light wind. Exposure to full sun or stagnant air can increase feel-like values by up to 15 degrees!
Everyday Examples: Sweating, Dew, and Rain
When we sweat to exhaust thermal energy by evaporation we aren’t actively grabbing the hottest water molecules, pulling then away from their neighbors, and throwing them into the gas phase. The evaporation happens spontaneously because thermal energy stored in water molecules that are stuck together is relatively concentrated compared to thermal energy stored in water molecules zipping around in the air and free to disperse. The transfer of thermal energy to the environment by evaporation is a spontaneous process because it increases the dispersion of energy throughout the system made up of you, the sweat, and the surrounding air.
When the relative humidity reaches 100% then evaporation has maximally dispersed the available thermal energy. Any additional evaporation would begin to over-concentrate energy in the air and decrease the overall level of energy dispersion. Therefore, we don’t see evaporation occurring once 100% humidity is reached. In fact if the humidity gets pushed above 100% (by a drop in air [temperature without a loss of water vapor) then energy is over-concentrated in the air and thus increasing dispersion of energy requires that water molecules come out of the vapor phase and condensation occurs spontaneously. When the liquid condenses on surfaces we call it dew, when the liquid condenses on particles in the air and falls to the ground we call it rain.
Exercises
Everyday Examples: Winter Dry Skin
The Pacific Northwest is famous for its winter rain, fog and general high humidity. However, people in the pacific northwest often suffer from dry skin in winter, but not summer when humidity is often less than 20 %. During winter, humid air is brought in from the outside and warmed by the heating system. That air still contains the same amount of water vapor, but is now at higher temperature, so the relative humidity is significantly reduced, even to the point of causing dry skin.
The Bends
We have learned that evaporation takes place even when a liquid isn’t boiling, so we may be wondering what causes boiling and how is it different from normal evaporation? Water ordinarily contains significant amounts of dissolved air and other impurities, which are observed as small bubbles of air in a glass of water. The bubbles formed within the water so the relative humidity inside the bubbles is 100%, meaning the maximum possible number of water molecules are inside the bubble as vapor. Those molecules collide with the walls of the bubble causing an outward pressure. The speed of the water molecules increases with temperature, so the pressure they exert does as well. At 100 °C the internal pressure exerted by the water vapor is equal to the atmospheric pressure trying to collapse the bubbles, so rather than collapse they will expand and rise, causing boiling. Once water is boiling, any additional thermal energy input goes into changing liquid water to water vapor, so the water will not increase temperature. Turning up the burner on the stove will not cook the food faster, it will just more quickly boil away (evaporate) the water.
Everyday Examples: The Bends
At high altitude the atmospheric pressure is lower, so molecules of water vapor don’t need to create as much pressure within bubbles to maintain boiling. Therefore, boiling will occur at a lower temperature and cooking foods by boiling will take longer. (Food packaging often gives alternative cook times for high altitude).
The same process is responsible for the bends, which refers to the formation of nitrogen bubbles within the blood upon rapid ascent while SCUBA diving. You might imaging that you could hang out underwater by breathing through a hose, and that would work in very shallow water. However, the high pressure exerted by water at depths below roughly 2 m (6 ft) would prevent the diaphragm and rib cage from expanding to pull air into the lungs. At greater depths you need to breath from a pressurized container which helps to force air into your lungs against the additional hydrostatic pressure. Of course if you breathed from the container at shallow depth then the pressure would be too high and would cause damage to your lungs. A pressure regulator that outputs the appropriate pressure according to the water depth is the core of the SCUBA system.
There is always some gas dissolved in your blood, including carbon dioxide, oxygen, and nitrogen. The amount of dissolved gas is determined by the temperature and the pressure. If temperature is high enough, and pressure is low enough, then boiling will occur. Breathing high pressure air from a SCUBA system while at depth forces these gases to dissolve into your blood in the amounts determined by your body temperature and the high pressure.
When ascending, the pressure drops quickly, but the body temperature stays constant, so the blood gases can begin to boil, starting with Nitrogen. There is not issue with blood temperature here, blood is still at body temperature, but the bubbles are a problem for the cardiovascular system. To prevent the bends, you must ascend slowly, allowing the gasses to slowly escape from the blood and be expelled in the breath, without forming large bubbles in the blood.
To treat the bends a patient is placed in a hyperbaric (high pressure) chamber. The high pressure collapses the bubbles and prevents new ones from forming. The pressure is then slowly decreases to allow the blood gasses to escape slowly, simulating a gradual ascent.

- Hyperthermia Patient by Mike Mitchell (photographer) [Public domain], via Wikimedia Commons ↵
- OpenStax University Physics, University Physics Volume 2. OpenStax CNX. Feb 6, 2019 http://cnx.org/contents/7a0f9770-1c44-4acd-9920-1cd9a99f2a1e@15.2 ↵
- OpenStax, Humidity, Evaporation, and Boiling. OpenStax CNX. Sep 9, 2013 http://cnx.org/contents/030347e9-f128-486f-a779-019ac474ff90@5 ↵
- "Zion National Park Visitor Center" by National Renewable Energy Laboratory, U.S. Department of Energy is in the Public Domain ↵
- "Heat Index" by National Weather Service, NOAA is in the Public Domain ↵
- "Decompression Chamber" by U.S. Navy Mass Communication Specialist 2nd Class Jayme Pastoric, is in the Public Domain. ↵
The condition of having a body temperature well above the normal range.
The condition of having a body temperature well below the normal range.
rate at which chemical potential energy is converted to thermal energy by the body, batteries, or heat engines. Also, rate at which thermal energy is converted to electrical energy by a thermal power plant.
A quantity representing the effect of applying a force to an object or system while it moves some distance.
Electromagnetic radiation spontaneously emitted by all objects with temperature above absolute zero.
An amount of thermal energy transferred due to a difference in temperature.
a two systems are in thermal equilibrium when they do not exchange heat, which means they must be at the same temperature
Thermal energy input required to change a unit mass of liquid into vapor.
relation between the amount of a material and the space it takes up, calculated as mass divided by volume.
a quantity of space, such as the volume within a box or the volume taken up by an object.
a measure of how many water molecules are in the vapor phase relative to the maximum number that could possibly be in the vapor phase at at a given temperature. A relative humidity of 100% means that no more water molecules can be added to the vapor phase.
Process of vapor changing phase into a liquid.
water that condenses on cool surfaces at night, when decreasing temperature forces humidity to 100% or higher
not changing, having the same value within a specified interval of time, space, or other physical variable

