65 Energy and Power
Energy
In this unit we will use energy conservation to study the balance of energy in the body and other systems. To do so, there are 5 types energy that we need to account for:
- kinetic energy: energy stored in the motion of objects (including the body and other objects that we move around)
- elastic (spring) potential energy: energy stored in the compression or tension of a material (including body tissues such as tendons and bones)
- gravitational potential energy: energy stored in a set of objects that are attracted by gravity (including objects that we lift farther from the Earth)
- chemical potential energy: energy stored in the chemicals bonds of a substance (including foods that we eat and fuels for vehicles)
- thermal energy: kinetic energy stored in the microscopic motion of atoms and molecules (including the atoms and molecules in your body and the environment)
Notice that thermal energy is actually just kinetic energy of tiny things, so overall we have two main categories of energy: Kinetic Energy (KE) and Potential Energy (PE). We can think of potential energy as energy that has the potential to become kinetic energy. For example, an object held up above the Earth has gravitational potential energy and a rubber band that is held stretched stores elastic potential energy. In either case, the potential energy is transferred to kinetic energy when we let go. When fuel is ignited then chemical potential energy transfers to kinetic energy in the atoms and molecules of the combustion products (thermal energy). If we are clever, we can then transfer that thermal energy into kinetic energy, which is exactly what combustion engines in vehicles are designed to do. The process of transferring energy from one form to another is known as work. Any time that work is done, energy must be transferred from one category of energy to another, and/or from one object to another, but the total energy of an isolated system can never change. The previous statement is the Principle of Energy Conservation and we should notice that it relies on the concept of a system, just like the as the Law of Conservation of Momentum.
Basic Metabolic Rate
The many small muscle actions accompanying all quiet activity, from sleeping to head scratching, all require a transfer of energy from one form to another, as do less visible muscle actions by the heart, lungs, and digestive tract. The rate at which energy is transferred is known as the Power. This power required to sustain life and to do different activities is called the metabolic rate and the power required for a person at rest is called the basal metabolic rate (BMR). The vast majority of this power is the transfer of chemical energy in food to thermal energy in the body, which is then exhausted to the environment.
| Organ | Power at rest (W) | Oxygen consumption (mL/min) | Percent of BMR |
| Liver & spleen | 23 | 67 | 27 |
| Brain | 16 | 47 | 19 |
| Skeletal muscle | 15 | 45 | 18 |
| Kidney | 9 | 26 | 10 |
| Heart | 6 | 17 | 7 |
| Other | 16 | 48 | 19 |
| Totals | 85 W | 250 mL/min | 100% |
At rest, the liver and spleen account for the largest fraction of the BMR, with the brain coming next. About 75% of the calories burned in a day go into these basic functions. A full 25% of all basal metabolic power of the body is used to maintain electrical potentials in all living cells. (Nerve cells use this electrical potential to generate nerve impulses.) This bioelectrical energy ultimately becomes mostly thermal energy, but some is utilized to power chemical processes such as in the kidneys and liver, and in fat production. The BMR is a function of age, gender, total body weight, and amount of muscle mass (because it requires more basic metabolic power than other body tissues).
Useful Work
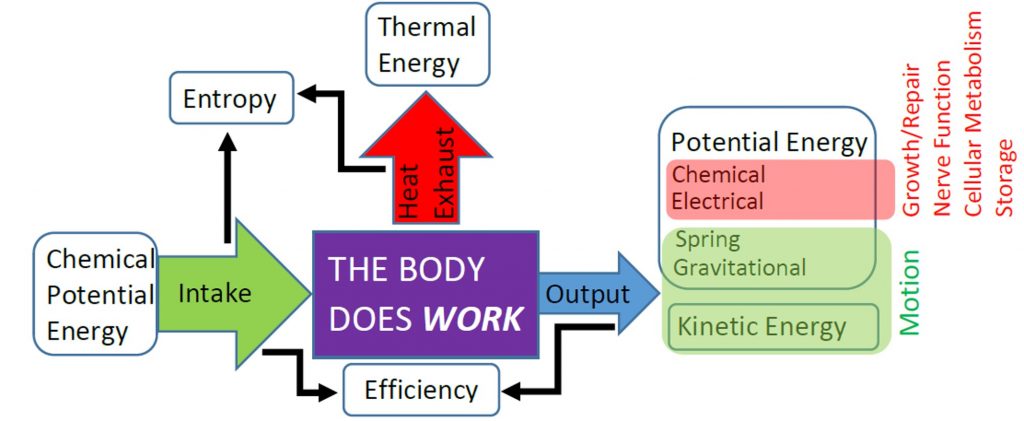
Of course, during vigorous exercise the metabolic power of the skeletal muscles and heart increases significantly because energy is then being transferred to potential or kinetic energy as well as thermal energy. For example, lifting a rubber ball increases gravitational potential energy, throwing the ball increases kinetic energy, and compressing the ball increases elastic potential energy. Those three forms of energy are known as mechanical energy, and work that transfers chemical potential energy to mechanical energy is known as useful work. Work that transfers chemical potential energy to thermal energy, such as all the work associated with the BMR, is not useful work. In the next chapter we will learn how to calculate the amount of useful work done by the body. The efficiency of both the body (and of engines and other machies) is determined by comparing the amount of chemical energy use to do useful work vs. not useful work. The following diagram summarizes the basic energetic functioning of the body, showing that the body does work to convert energy from one form to another, but that energy is not really “used up” by the body.
- Significant content in this chapter was adapted from: OpenStax, College Physics. OpenStax CNX. Aug 30, 2019 http://cnx.org/contents/031da8d3-b525-429c-80cf-6c8ed997733a@17.22 ↵
A quantity representing the effect of applying a force to an object or system while it moves some distance.
Energy cannot be created or destroyed, only transferred through the process of work
The total momentum of an isolated system cannot change.
rate of energy conversion or transfer
work done by the body to transfer chemical potential energy to mechanical energy (kinetic energy, gravitational potential energy, elastic potential energy)

