80 Thermodynamic Limits on Efficiency
The First Law of Thermodynamics
The amount of thermal energy transferred between two systems due a difference in their temperature is known as Heat(Q). The units for heat are the same as for thermal energy because the heat is just the amount of thermal energy that was transferred. The Law of Conservation of Energy tells us that the total energy of an isolated system cannot change. Therefore, the internal energy stored within the body (![]() ) must decrease when the body exhausts heat to the environment or when work is done by the body (
) must decrease when the body exhausts heat to the environment or when work is done by the body (![]() ) on the environment. Written in those terms the Law of Conservation of Energy is renamed the First Law of Thermodynamics, but it’s really the same thing, and just like the Law of Conservation of Energy, it applies to everything, not just the body.
) on the environment. Written in those terms the Law of Conservation of Energy is renamed the First Law of Thermodynamics, but it’s really the same thing, and just like the Law of Conservation of Energy, it applies to everything, not just the body.
![]()
Notice that the First Law of Thermodynamics also covers the case when negative work is done by the body, as when kinetic energy is dissipated as thermal energy within muscles during a landing. In that case the negative in the First Law equation cancels out the negative work to give an overall positive, meaning that the internal energy of the body increased which makes sense because it now has more thermal energy.
Engines
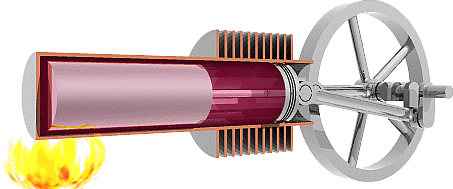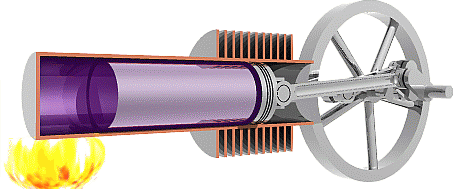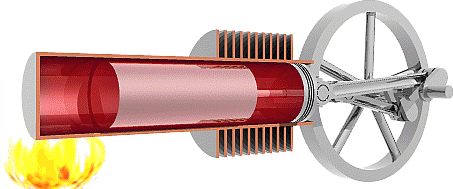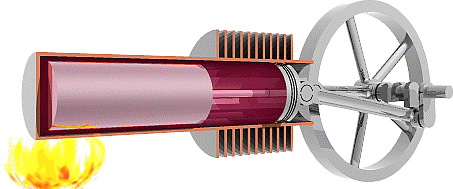
We are now ready to model the human body as an engine that takes chemical potential energy (food) as input and then does useful work to transfer some of that energy to the environment as mechanical energy while the remainder becomes exhaust heat that was not used to do useful work. Classical heat engines like the combustion engines in cars also exhausts heat, but they use thermal energy as the input instead of chemical potential energy. That thermal energy sometimes comes from burning a chemical fuel, as with combustion engines in cars, but it may also come from hot fluids warmed by the Earth (geothermal) or by the sun (solar-thermal), or from other more exotic sources.
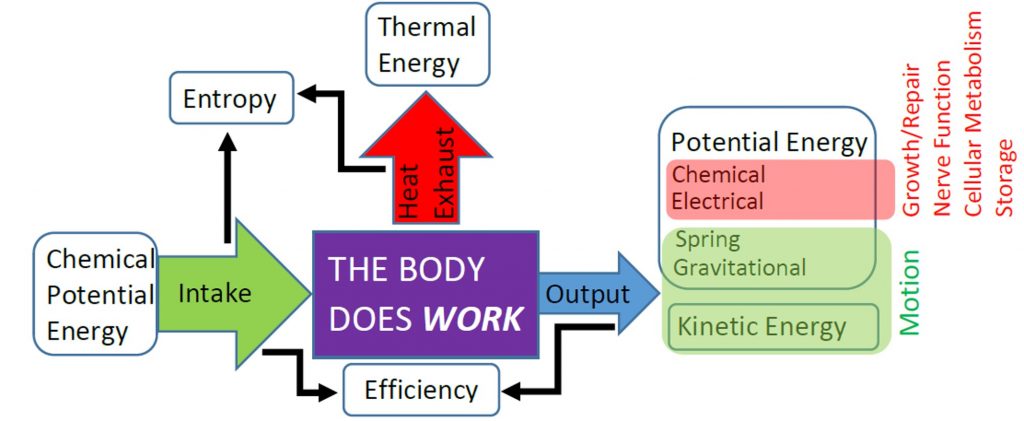
In previous units and chapters we have studied the mechanical efficiency of the body as the ratio of mechanical (useful) work done to chemical energy used, but we have not specified why the efficiency is so low. Energy Conservation, or the First Law of Thermodynamics, only ensures that the efficiency cannot be greater than 100%, so the 20% efficiency of the body and other typical engines seems really low. However, the efficiency of the body and other engines is limited even further by the of the Second Law of Thermodynamics.
The Second Law of Thermodynamics requires the heat exhausted is always greater than zero, so some input energy is not available to do work. In other words, the work done must always be less than the input energy and efficiency will always be less than 100%. To understand why the Second Law requires that engines must exhaust heat we first need to examine the Second Law in detail.
The second Law of Thermodynamics
The Second Law of Thermodynamics states that any real process increases the total dispersion of energy throughout the universe. We define Entropy (S) as measure of how well and how widely energy is dispersed through a system, so we can reword the Second Law to say any real process increases the total entropy of the universe. For example, we know that higher temperature means a greater average thermal energy per molecule, so we can think of temperature as a measure how concentrated thermal energy is within a system. If we consider a cold room containing a warm object as our system, then the thermal energy in our system is not very well dispersed because there’s a higher concentration of thermal energy in the warm object. The Second Law of thermodynamics predicts that thermal energy should move from the warm body to the cold environment to better disperse the energy throughout the system and increase the entropy of the universe, and that is exactly what we observe. That process will continue until the concentration of thermal energy in the room is the same as in the object, which means the object and the room would have the same temperature and we say the system has reached thermal equilibrium.
Reinforcement Exercises
Everyday Examples: Limits on Human Mechanical Efficiency
Muscle contraction relies on the release of chemical potential energy stored in ATP molecules. Before contraction, that energy is concentrated into certain molecules in certain areas of a muscle cell. When that energy is released, the entropy of the molecules decreases, so the entropy of their environment must increase by more, and this is achieved because most of the energy released from the ATP molecules is degraded to heat and distributed to the environment. After contraction, a muscle cell must be reorganized, which decreases entropy, but we know overall entropy must increase for any real process, so some additional thermal energy must be dispersed to the environment during reorganization in order to provide the necessary entropy increase. All of this exhausted thermal energy is “wasted” because it is came from stored chemical potential energy, but is no longer available for the body to use in doing useful work. Therefore, entropy and the Second Law of Thermodynamics limit the efficiency of the human body.[1]
An amount of thermal energy transferred due to a difference in temperature.
A measure of energy dispersion in a system.
A quantity representing the effect of applying a force to an object or system while it moves some distance.

