61 Changes in Enzyme Activity
It would seem ideal to have a scenario in which all of an organism’s enzymes existed in abundant supply and functioned optimally under all cellular conditions, in all cells, at all times. However, this is not true for a variety of reasons. First, it would require a lot of energy to produce all an organism’s enzymes all the time. Also, cellular needs and conditions constantly vary from cell to cell, and change within individual cells over time. The required enzymes of stomach cells differ from those of fat storage cells, skin cells, blood cells, and nerve cells. Furthermore, a digestive organ cell works much harder to process and break down nutrients during the time that closely follows a meal compared with many hours after a meal. As these cellular demands and conditions vary, so must the amounts and functionality of different enzymes.
Since the rates of biochemical reactions are controlled by activation energy, and enzymes lower and determine activation energies for chemical reactions, the relative amounts and functioning of the variety of enzymes within a cell ultimately determine which reactions will proceed and at what rates. This determination is tightly controlled in cells.
Regulation
Enzymes can also be regulated in ways that either promote or reduce enzyme activity. There are many kinds of molecules that inhibit or promote enzyme function, and various mechanisms by which they do so.
- In some cases of enzyme inhibition, an inhibitor molecule is similar enough to a substrate that it can bind to the active site and simply block the substrate from binding.
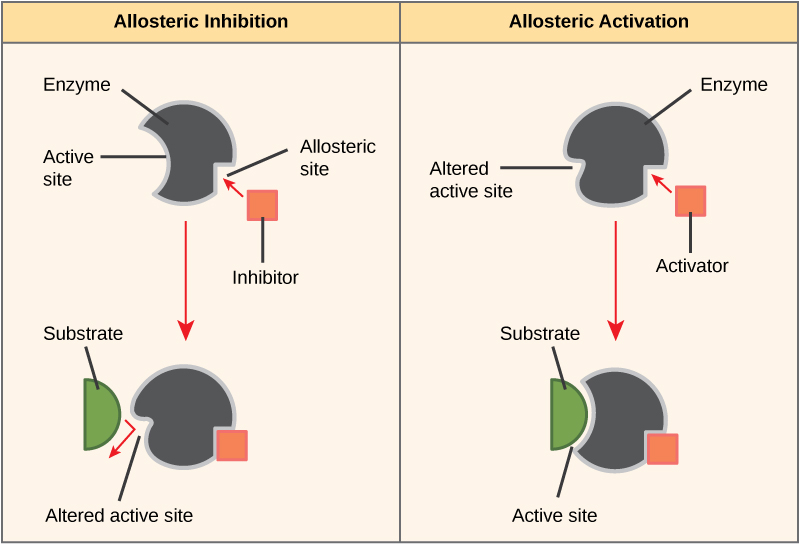
- In other cases, an inhibitor molecule binds to the enzyme in a location other than the active site, called an allosteric site, but still manages to block substrate binding to the active site (Figure 4).
- Some inhibitor molecules bind to enzymes in a location where their binding causes a change in the shape of the enzyme that makes the enzyme less likely to bind to its substrate.
- There are also activator molecules that can increase the ability of an enzyme to bind to its substrate.
Cofactors and Coenzymes
Many enzymes do not work optimally, or even at all, unless bound to other specific non-protein helper molecules. They may bond either temporarily through ionic or hydrogen bonds, or permanently through stronger covalent bonds. Binding to these molecules promotes optimal shape and function of their respective enzymes – they activate the enzyme. Two examples of these types of helper molecules are cofactors and coenzymes. Cofactors are inorganic ions such as ions of iron and magnesium. Coenzymes are organic helper molecules, those with a basic atomic structure made up of carbon and hydrogen. Like enzymes, these molecules participate in reactions without being changed themselves and are ultimately recycled and reused. Vitamins are the source of coenzymes. Some vitamins are the precursors of coenzymes and others act directly as coenzymes. Vitamin C is a direct coenzyme for multiple enzymes that take part in building the important connective tissue, collagen. Therefore, enzyme function is, in part, regulated by the abundance of various cofactors and coenzymes, which may be supplied by an organism’s diet or, in some cases, produced by the organism.
Effect of environmental conditions
Enzyme activity is subject to influences of the local environment. In a cold environment, enzymes function more slowly because the molecules are moving more slowly. The substrate bumps into the enzyme less frequently. As the temperature increases, molecules move more quickly, so the enzyme functions at a higher rate. Increasing temperature generally increases reaction rates, enzyme-catalyzed or otherwise. You may have noticed that sugar dissolves faster in hot coffee than in cold ice tea – this is because the molecules are moving more quickly in hot coffee, which increases the rate of the reaction. However, temperatures that are too high will reduce the rate at which an enzyme catalyzes a reaction. This is because hot temperatures will eventually cause the enzyme to denature, an irreversible change in the three-dimensional shape and therefore the function of the enzyme (Figure 5).
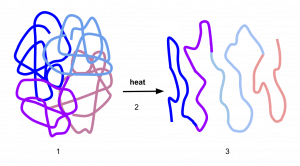
Denaturation is caused by the breaking of the bonds that hold the enzyme together in its three-dimensional shape. Heat can break hydrogen and ionic bonds, which disrupts the shape of the enzyme and will change the shape of the active site. Cold temperatures do not denature enzymes because cold does not cause chemical bonds to break.

Enzymes are suited to function best within a certain temperature, pH, and salt concentration range. In addition to high temperatures, extreme pH and salt concentrations can cause enzymes to denature. Both acidic and basic pH can cause enzymes to denature because the presence of extra H+ ions (in an acidic solution) or OH- ions (in a basic solution) can modify the chemical structure of the amino acids forming the protein, which can cause the chemical bonds holding the three-dimensional structure of the protein to break. High salt concentrations can also cause chemical bonds within the protein to break in a similar matter.
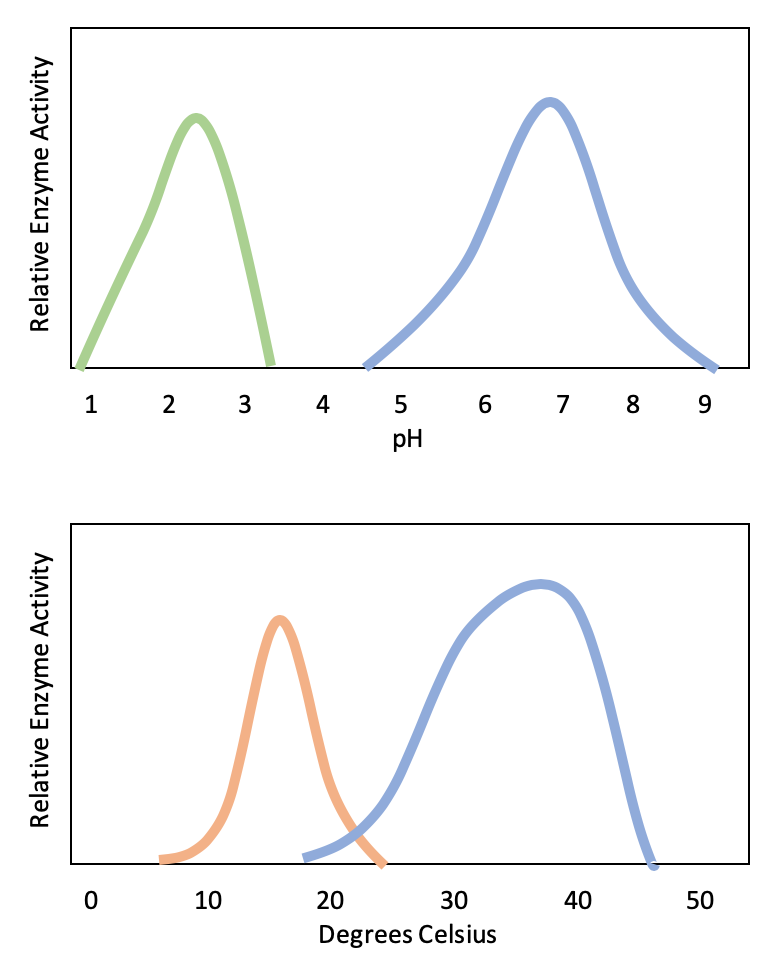
Typically, enzymes function optimally in the environment where they are typically found and used. For example, the enzyme amylase is found in saliva, where it functions to break down starch (a polysaccharide – carbohydrate chain) into smaller sugars. Note that in this example, amylase is the enzyme, starch is the substrate, and smaller sugars are the product. The pH of saliva is typically between 6.2 and 7.6, with roughly 6.7 being the average. The optimum pH of amylase is between 6.7 and 7.0, which is close to neutral (Figure 3). The optimum temperature for amylase is close to 37ºC (which is human body temperature).
References
Unless otherwise noted, images on this page are licensed under CC-BY 4.0 by OpenStax.
Text adapted from: OpenStax, Concepts of Biology. OpenStax CNX. May 18, 2016 http://cnx.org/contents/b3c1e1d2-839c-42b0-a314-e119a8aafbdd@9.10

