76 Passive Transport: Diffusion
The most direct forms of membrane transport are passive. Passive transport is a naturally occurring phenomenon and does not require the cell to expend energy to accomplish the movement. In passive transport, substances move from an area of higher concentration to an area of lower concentration in a process called diffusion. A physical space in which there is a different concentration of a single substance is said to have a concentration gradient.
Diffusion
Diffusion is a passive process of transport. A single substance tends to move from an area of high concentration to an area of low concentration until the concentration is equal across the space. You are familiar with diffusion of substances through the air. For example, think about someone opening a bottle of perfume in a room filled with people. The perfume is at its highest concentration in the bottle and is at its lowest at the edges of the room. The perfume vapor will diffuse, or spread away, from the bottle, and gradually, more and more people will smell the perfume as it spreads. Materials move within the cell’s cytosol by diffusion, and certain materials move through the plasma membrane by diffusion (Figure 1). Diffusion expends no energy. Rather the different concentrations of materials in different areas are a form of potential energy, and diffusion is the dissipation of that potential energy as materials move down their concentration gradients, from high to low.
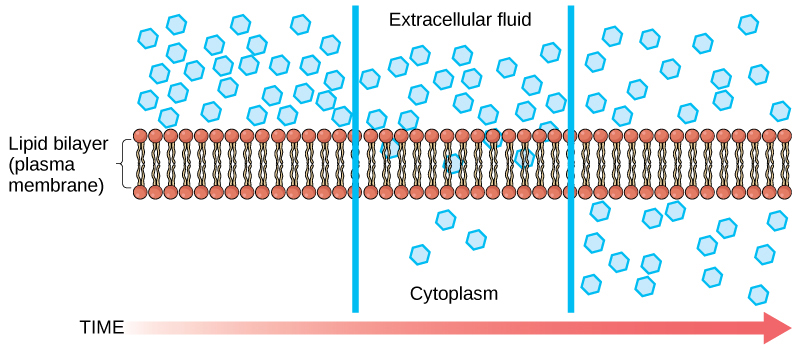
Each separate substance in a medium, such as the extracellular fluid, has its own concentration gradient, independent of the concentration gradients of other materials. Additionally, each substance will diffuse according to that gradient.
Several factors affect the rate of diffusion:
- Extent of the concentration gradient: The greater the difference in concentration, the more rapid the diffusion. The closer the distribution of the material gets to equilibrium, the slower the rate of diffusion becomes.
- Mass of the molecules diffusing: More massive molecules move more slowly, because it is more difficult for them to move between the molecules of the substance they are moving through; therefore, they diffuse more slowly.
- Temperature: Higher temperatures increase the energy and therefore the movement of the molecules, increasing the rate of diffusion.
- Solvent density: As the density of the solvent increases, the rate of diffusion decreases. The molecules slow down because they have a more difficult time getting through the denser medium.
Gas Exchange
Our bodies need to bring in oxygen and get rid of excess carbon dioxide. This process is called gas exchange. Gas exchange occurs at two sites in the body:
- In the lungs, where oxygen is picked up and carbon dioxide is released at the respiratory membrane. This is called external respiration.
- At the tissues, where oxygen is released and carbon dioxide is picked up. This is called internal respiration.
The actual exchange of gases occurs due to simple diffusion, which means that energy is not required to move oxygen or carbon dioxide across membranes. Instead, these gases follow pressure gradients that allow them to diffuse. You’ll learn more about partial pressure and pressure gradients in A&P. Pressure is proportional to concentration, so we will discuss this process by talking about the concentration of gas, rather than its pressure. The anatomy of the lung maximizes the diffusion of gases: The respiratory membrane is highly permeable to gases; the respiratory and blood capillary membranes are very thin; and there is a large surface area throughout the lungs.
External Respiration
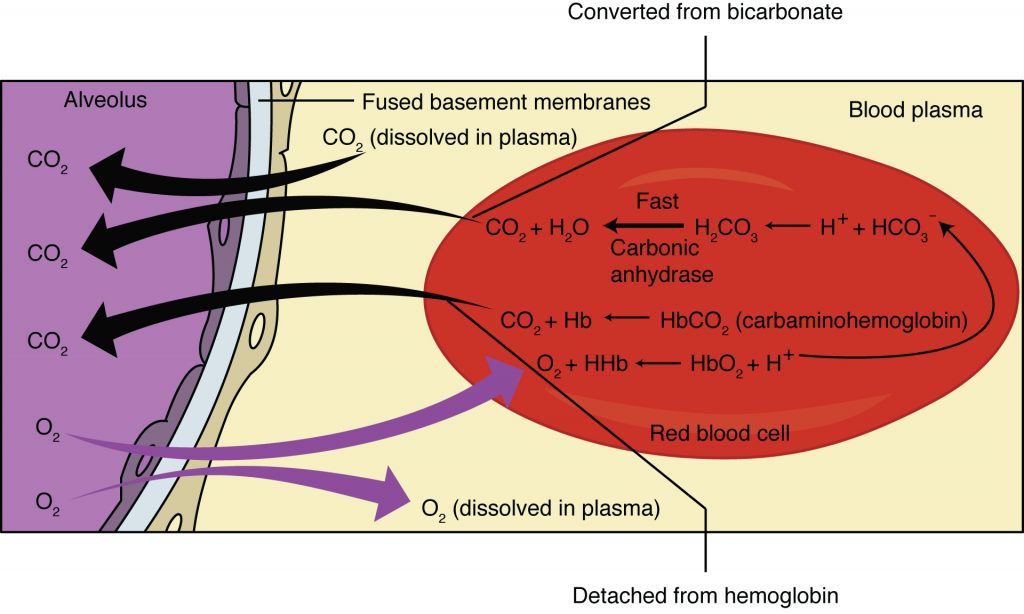
As blood is pumped through small blood vessels (capillaries) in the lungs, gas exchange occurs from the air in the alveoli into the blood. Although a small amount of the oxygen is able to dissolve directly into plasma from the alveoli, most of the oxygen is picked up by erythrocytes (red blood cells) and binds to a protein called hemoglobin. Oxygenated hemoglobin is red, causing the overall appearance of bright red oxygenated blood, which returns to the heart through the pulmonary veins. Carbon dioxide is released in the opposite direction of oxygen, from the blood to the alveoli.
Oxygen is diffusing from the air inside the alveoli within the lungs into the erythrocytes and blood plasma. Diffusion is a type of passive transport, where molecules move from high concentration to low concentration. This means that the concentration of oxygen in the air must be higher than it is in the blood.
The concentration of carbon dioxide is also different between the alveolar air and the blood of the capillary. Carbon dioxide diffuses out of the blood into the air spaces because the concentration of carbon dioxide is higher in the blood than in the air spaces.
Internal Respiration
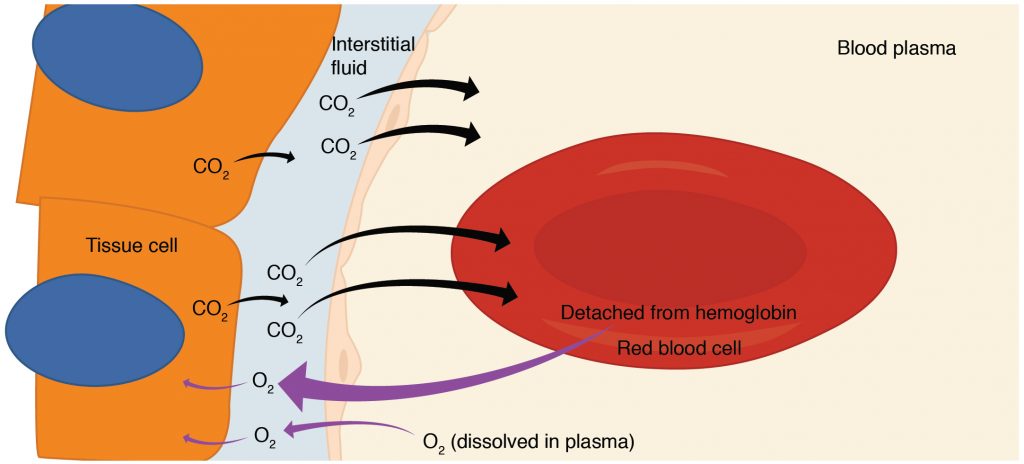
Internal respiration is gas exchange that occurs at the level of body tissues (Figure 2). Similar to external respiration, internal respiration also occurs as simple diffusion due to a concentration gradient. However, the concentrations are opposite of those present at the respiratory membrane. Oxygen diffuses into tissues because its concentration is higher in the blood than inside the tissue (where oxygen is always being used up during cellular respiration). Carbon dioxide, which is always being produced during cellular respiration, diffuses out for the opposite reason. Hemoglobin that has little oxygen bound to it loses much of its brightness, so that blood returning to the heart is more burgundy in color. The blood is then pumped back to the lungs to be oxygenated once again during external respiration.
References
Unless otherwise noted, images on this page are licensed under CC-BY 4.0 by OpenStax.
Text adapted from: OpenStax, Concepts of Biology. OpenStax CNX. May 18, 2016 http://cnx.org/contents/b3c1e1d2-839c-42b0-a314-e119a8aafbdd@9.10
Gas Exchange Textbook from: OpenStax, Anatomy and Physiology. OpenStax CNX. August 13, 2019. https://cnx.org/contents/FPtK1zmh@16.1:mFGdwqYB@12/22-4-Gas-Exchange

