Free and Activation Energy
After learning that chemical reactions release energy when energy-storing bonds are broken, an important next question is the following: How is the energy associated with these chemical reactions quantified and expressed? How can the energy released from one reaction be compared to that of another reaction? A measurement of free energy is used to quantify these energy transfers. Recall that according to the second law of thermodynamics, all energy transfers involve the loss of some amount of energy in an unusable form such as heat. Free energy specifically refers to the energy associated with a chemical reaction that is available after the losses are accounted for. In other words, free energy is usable energy, or energy that is available to do work. Looking at this concept in a biological sense, free energy is the energy within a molecule that can be used to perform work. Glucose has a lot of free energy because there is a lot of energy stored within the bonds of the glucose molecule. Carbon dioxide has a much lower free energy because there is much less energy stored in its bonds.
If energy is released during a chemical reaction, then the change in free energy from the conversion of the reactants to the products, signified as ΔG (delta G) will be a negative number. A negative change in free energy also means that the products of the reaction have less free energy than the reactants, because they release some free energy during the reaction. Reactions that have a negative change in free energy and consequently release free energy are called exergonic reactions. Think: exergonic means energy is exiting the system. These reactions are also referred to as spontaneous reactions, and their products have less stored energy than the reactants. An important distinction must be drawn between the term spontaneous and the idea of a chemical reaction occurring immediately. Contrary to the everyday use of the term, a spontaneous reaction is not one that suddenly or quickly occurs. The rusting of iron is an example of a spontaneous reaction that occurs slowly, little by little, over time.
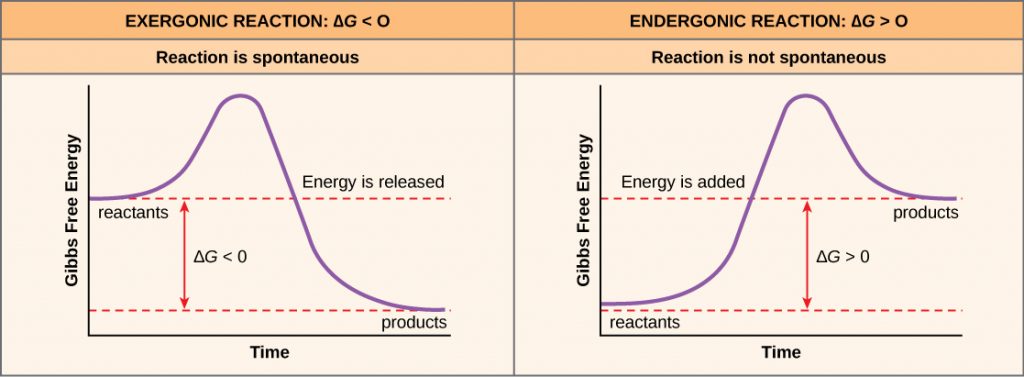
If a chemical reaction absorbs energy rather than releases energy on balance, then the ΔG for that reaction will be a positive value. In this case, the products have more free energy than the reactants. Thus, the products of these reactions can be thought of as energy-storing molecules. These chemical reactions are called endergonic reactions and they are nonspontaneous.
An endergonic reaction will not take place on its own without the addition of free energy.

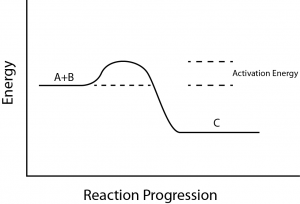
There is another important concept that must be considered regarding endergonic and exergonic reactions. Exergonic reactions require a small amount of energy input to get going, before they can proceed with their energy-releasing steps.
These reactions have a net release of energy, but still require some energy input in the beginning. This small amount of energy input necessary for all chemical reactions to occur is called the activation energy (Figure 3).
References
Unless otherwise noted, images on this page are licensed under CC-BY 4.0 by OpenStax.
Text adapted from: OpenStax, Concepts of Biology. OpenStax CNX. May 18, 2016 http://cnx.org/contents/b3c1e1d2-839c-42b0-a314-e119a8aafbdd@9.10

