Metabolism
An organism’s metabolism is the sum total of all the chemical reactions that occur within the organism. These chemical reactions fall into two basic categories:
- Anabolism: building polymers (large molecules that the cell needs).
- Catabolism: breaking down polymers to release energy.
This means that metabolism is composed of synthesis (anabolism) and degradation (catabolism) (Figure 1).
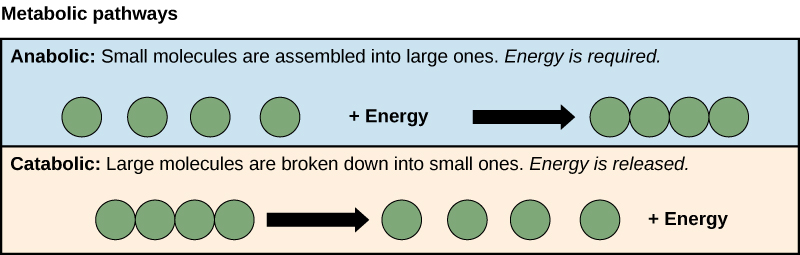
It is important to know that the chemical reactions of metabolic pathways do not take place on their own. Each reaction step is facilitated, or catalyzed, by a protein called an enzyme. Enzymes are important for catalyzing all types of biological reactions—those that require energy as well as those that release energy. Refer back to the chapter on enzymes if you need a reminder about this topic.
Consider the metabolism of sugar (a carbohydrate). This is a classic example of one of the many cellular processes that use and produce energy. Living things consume sugars as a major energy source, because sugar molecules have a great deal of energy stored within their bonds. For the most part, photosynthesizing organisms like plants produce these sugars. During photosynthesis, plants use energy (originally from sunlight) to convert carbon dioxide gas (CO2) into sugar molecules (like glucose: C6H12O6). They consume carbon dioxide and produce oxygen as a waste product. This reaction is summarized as:
6CO2 + 6H2O–>C6H12O6 + 6O2
Recall from chemistry that the abbreviation “CO2” means “one carbon atom covalently bonded to two oxygen atoms.” Water, “H2O” is two hydrogen atoms covalently bonded to one oxygen atom. And “C6H12O6” has 6 carbon atoms, 12 hydrogen atoms, and 6 oxygen atoms that are covalently bonded together.

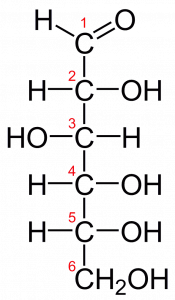
The process of producing glucose from carbon dioxide and water requires an energy input to proceed because glucose contains more energy in its molecular bonds than carbon dioxide does.
In contrast, energy-storage molecules such as glucose are consumed to be broken down to use their energy. The reaction that harvests the energy of a sugar molecule in cells requiring oxygen to survive can be summarized by the reverse reaction to photosynthesis. In this reaction, oxygen is consumed and carbon dioxide is released as a waste product. The reaction is summarized as:
C6H12O6 + 6O2–>6H2O + 6CO2
Both of these reactions involve many steps.
The processes of making and breaking down sugar molecules illustrate two examples of metabolic pathways. A metabolic pathway is a series of chemical reactions that takes a starting molecule and modifies it, step-by-step, through a series of metabolic intermediates, eventually yielding a final product. In the example of sugar metabolism, the first metabolic pathway synthesized sugar from smaller molecules, and the other pathway broke sugar down into smaller molecules.
References
Unless otherwise noted, images on this page are licensed under CC-BY 4.0 by OpenStax.
Text adapted from: OpenStax, Concepts of Biology. OpenStax CNX. May 18, 2016 http://cnx.org/contents/b3c1e1d2-839c-42b0-a314-e119a8aafbdd@9.10

