Passive Transport: Diffusion
The most direct forms of membrane transport are passive. Passive transport is a naturally occurring phenomenon and does not require the cell to expend energy to accomplish the movement. In passive transport, substances move from an area of higher concentration to an area of lower concentration in a process called diffusion. A physical space in which there is a different concentration of a single substance is said to have a concentration gradient.
Diffusion
Diffusion is a passive process of transport. A single substance tends to move from an area of high concentration to an area of low concentration until the concentration is equal across the space. You are familiar with diffusion of substances through the air. For example, think about someone opening a bottle of perfume in a room filled with people. The perfume is at its highest concentration in the bottle and is at its lowest at the edges of the room. The perfume vapor will diffuse, or spread away, from the bottle, and gradually, more and more people will smell the perfume as it spreads. Materials move within the cell’s cytosol by diffusion, and certain materials move through the plasma membrane by diffusion (Figure 1). Diffusion expends no energy. Rather the different concentrations of materials in different areas are a form of potential energy, and diffusion is the dissipation of that potential energy as materials move down their concentration gradients, from high to low.
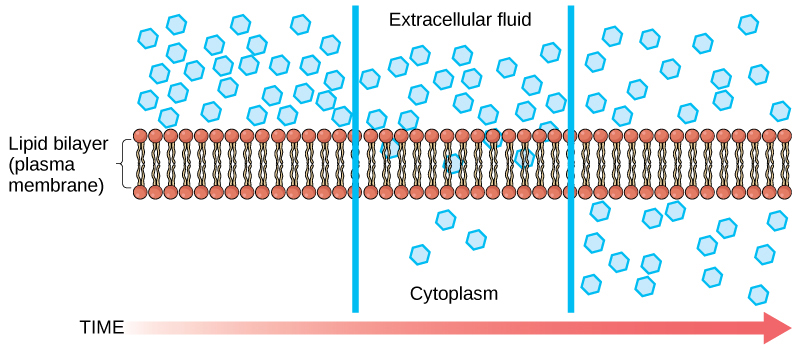
Each separate substance in a medium, such as the extracellular fluid, has its own concentration gradient, independent of the concentration gradients of other materials. Additionally, each substance will diffuse according to that gradient.
Several factors affect the rate of diffusion:
- Extent of the concentration gradient: The greater the difference in concentration, the more rapid the diffusion. The closer the distribution of the material gets to equilibrium, the slower the rate of diffusion becomes.
- Mass of the molecules diffusing: More massive molecules move more slowly, because it is more difficult for them to move between the molecules of the substance they are moving through; therefore, they diffuse more slowly.
- Temperature: Higher temperatures increase the energy and therefore the movement of the molecules, increasing the rate of diffusion.
- Solvent density: As the density of the solvent increases, the rate of diffusion decreases. The molecules slow down because they have a more difficult time getting through the denser medium.
References
Unless otherwise noted, images on this page are licensed under CC-BY 4.0 by OpenStax.
Text adapted from: OpenStax, Concepts of Biology. OpenStax CNX. May 18, 2016 http://cnx.org/contents/b3c1e1d2-839c-42b0-a314-e119a8aafbdd@9.10

