Metabolism without Oxygen: Fermentation
In aerobic respiration, the final electron acceptor for the electron transport chain is an oxygen molecule, O2. If aerobic respiration occurs, then approximately 30 molecules of ATP will be produced during the electron transport chain and chemiosmosis using the energy of the high-energy electrons carried by NADH or FADH2 to the electron transport chain. When NADH or FADH2 give their high energy electrons to the electron transport chain, NAD+ and FAD are regenerated. These low energy molecules cycle back to glycolysis and/or the citric acid cycle, where they pick up more high energy electrons and allow the process to continue.
Glycolysis and the citric acid cycle can not occur if there is not NAD+ present to pick up electrons as the reactions proceed. When oxygen is present, this isn’t a problem – all of the NADH and FADH2 that were produced during glycolysis and the citric acid cycle are converted back into NAD+ and FAD after the electron transport chain. When no oxygen is present, the electron transport chain can’t run because there is no oxygen to act as the final electron acceptor. This means that the ETC will not be accepting electrons from NADH as its source of power, so NAD+ will not be regenerated. Both glycolysis and the citric acid cycle require NAD+ to accept electrons during their chemical reactions. In order for the cell to continue to generate any ATP, NADH must be converted back to NAD+ for use as an electron carrier. Anaerobic processes use different mechanisms, but all function to convert NAD+ back into NADH.
How is this done?
- Processes that use an organic molecule to regenerate NAD+ from NADH are collectively referred to as fermentation.
- In contrast, some living systems use an inorganic molecule (such as nitrate or sulfur) to regenerate NAD+.
Both of these methods are called anaerobic cellular respiration. They do not require oxygen to achieve NAD+ regeneration and enable organisms to convert energy for their use in the absence of oxygen.
During anaerobic respiration, only glycolysis occurs. The 2 molecules of NADH that are generated during glycolysis are then converted back into NAD+ during anaerobic respiration so that glycolysis can continue. Since glycolysis only produces 2 ATP, anaerobic respiration is much less efficient than aerobic respiration (2 ATP molecules compared to 36-ish ATP molecules). However, 2 ATP molecules is much better for a cell than 0 ATP molecules. In anaerobic situations, the cell needs to continue performing glycolysis to generate 2 ATP per glucose because if a cell is not generating any ATP, it will die.
Note that the only part of aerobic respiration that physically uses oxygen is the electron transport chain. However, the citric acid cycle can not occur in the absence of oxygen because there is no way to regenerate the NAD+ used during this process.
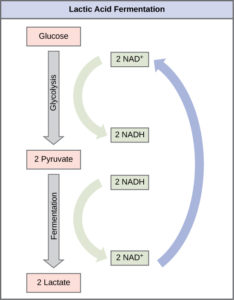
Lactic Acid Fermentation
The fermentation method used by animals and some bacteria like those in yogurt is lactic acid fermentation (Figure 1). This occurs routinely in mammalian red blood cells and in skeletal muscle that does not have enough oxygen to allow aerobic respiration to continue (such as in muscles after hard exercise). The chemical reaction of lactic acid fermentation is the following:
The build-up of lactic acid causes muscle stiffness and fatigue. In muscles, lactic acid produced by fermentation must be removed by the blood circulation and brought to the liver for further metabolism. Once the lactic acid has been removed from the muscle and is circulated to the liver, it can be converted back to pyruvic acid and further catabolized (broken down) for energy.
Note that the purpose of this process is not to produce lactic acid (which is a waste product and is excreted from the body). The purpose is to convert NADH back into NAD+ so that glycolysis can continue so that the cell can produce 2 ATP per glucose.
Alcohol Fermentation
Another familiar fermentation process is alcohol fermentation (Figure 2), which produces ethanol, an alcohol. The alcohol fermentation reaction is the following:
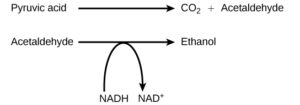
The fermentation of pyruvic acid by yeast produces the ethanol found in alcoholic beverages (Figure 3). If the carbon dioxide produced by the reaction is not vented from the fermentation chamber, for example in beer and sparkling wines, it remains dissolved in the medium until the pressure is released. Ethanol above 12 percent is toxic to yeast, so natural levels of alcohol in wine occur at a maximum of 12 percent.

Again, the purpose of this process is not to produce ethanol, but rather to convert NADH back into NAD+ so that glycolysis can continue.
References
Unless otherwise noted, images on this page are licensed under CC-BY 4.0 by OpenStax.
Text adapted from: OpenStax, Concepts of Biology. OpenStax CNX. May 18, 2016 http://cnx.org/contents/b3c1e1d2-839c-42b0-a314-e119a8aafbdd@9.10

