Errors in Meiosis
Disorders in Chromosome Number
The isolation and microscopic observation of chromosomes forms the basis of cytogenetics and is the primary method by which clinicians detect chromosomal abnormalities in humans. A karyotype is the number and appearance of chromosomes, including their length, banding pattern, and centromere position. To obtain a view of an individual’s karyotype, cytologists photograph the chromosomes and then cut and paste each chromosome into a chart, or karyogram (Figure 1).
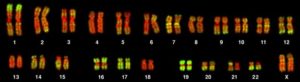
By observing a karyogram, geneticists can actually visualize the chromosomal composition of an individual to confirm or predict genetic abnormalities in offspring even before birth.
Geneticists Use Karyograms to Identify Chromosomal Aberrations
Although Mendel is referred to as the “father of modern genetics,” he performed his experiments with none of the tools that the geneticists of today routinely employ. One such powerful cytological technique is karyotyping, a method in which traits characterized by chromosomal abnormalities can be identified from a single cell. To observe an individual’s karyotype, a person’s cells (like white blood cells) are first collected from a blood sample or other tissue. In the laboratory, the isolated cells are stimulated to begin actively dividing. A chemical called colchicine is then applied to cells to arrest condensed chromosomes in metaphase. Cells are then made to swell using a hypotonic solution so the chromosomes spread apart. Finally, the sample is preserved in a fixative and applied to a slide.
The geneticist then stains chromosomes with one of several dyes to better visualize the distinct and reproducible banding patterns of each chromosome pair. Following staining, the chromosomes are viewed using bright-field microscopy. A common stain choice is the Giemsa stain. Giemsa staining results in approximately 400–800 bands (of tightly coiled DNA and condensed proteins) arranged along all of the 23 chromosome pairs; an experienced geneticist can identify each band. In addition to the banding patterns, chromosomes are further identified on the basis of size and centromere location. To obtain the classic depiction of the karyotype in which homologous pairs of chromosomes are aligned in numerical order from longest to shortest, the geneticist obtains a digital image, identifies each chromosome, and manually arranges the chromosomes into this pattern (Figure 1).
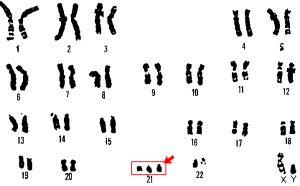
At its most basic, the karyogram may reveal genetic abnormalities in which an individual has too many or too few chromosomes per cell. Examples of this are Down Syndrome, which is identified by a third copy of chromosome 21, and Turner Syndrome, which is characterized by the presence of only one X chromosome in women instead of the normal two. Geneticists can also identify large deletions or insertions of DNA. For instance, Jacobsen Syndrome—which involves distinctive facial features as well as heart and bleeding defects—is identified by a deletion on chromosome 11. Finally, the karyotype can pinpoint translocations, which occur when a segment of genetic material breaks from one chromosome and reattaches to another chromosome or to a different part of the same chromosome. Translocations are implicated in certain cancers, including chronic myelogenous leukemia.
During Mendel’s lifetime, inheritance was an abstract concept that could only be inferred by performing crosses and observing the traits expressed by offspring. By observing a karyogram, today’s geneticists can actually visualize the chromosomal composition of an individual to confirm or predict genetic abnormalities in offspring, even before birth.
Of all the chromosomal disorders, abnormalities in chromosome number are the most easily identifiable from a karyogram. Disorders of chromosome number include the duplication or loss of entire chromosomes, as well as changes in the number of complete sets of chromosomes. They are caused by nondisjunction, which occurs when pairs of homologous chromosomes or sister chromatids fail to separate during meiosis. The risk of nondisjunction increases with the age of the parents.
Nondisjunction can occur during either meiosis I or II, with different results (Figure 2). If homologous chromosomes fail to separate during meiosis I, the result is two gametes that lack that chromosome and two gametes with two copies of the chromosome. If sister chromatids fail to separate during meiosis II, the result is one gamete that lacks that chromosome, two normal gametes with one copy of the chromosome, and one gamete with two copies of the chromosome.
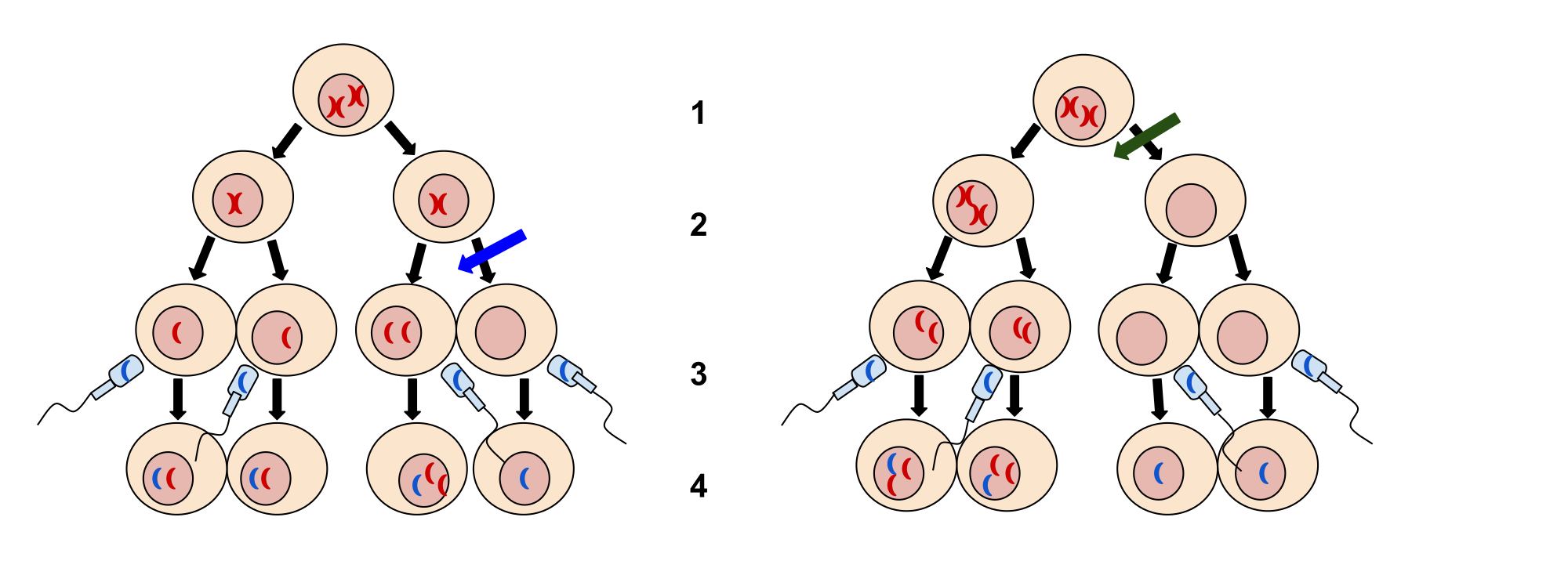
An individual with the appropriate number of chromosomes for their species is called euploid; in humans, euploidy corresponds to 22 pairs of autosomes and one pair of sex chromosomes (such as is seen in the karyotype in Figure 1). An individual with an error in chromosome number is described as aneuploid, a term that includes monosomy (loss of one chromosome) or trisomy (gain of an extraneous chromosome). Monosomic human zygotes missing any one copy of an autosome invariably fail to develop to birth because they have only one copy of essential genes. Most autosomal trisomies also fail to develop to birth; however, duplications of some of the smaller chromosomes (13, 15, 18, 21, or 22) can result in offspring that survive for several weeks to many years. Trisomic individuals suffer from a different type of genetic imbalance: an excess in gene dose. Cell functions are calibrated to the amount of gene product produced by two copies (doses) of each gene; adding a third copy (dose) disrupts this balance. The most common trisomy is that of chromosome 21, which leads to Down syndrome. Individuals with this inherited disorder have characteristic physical features and developmental delays in growth and cognition.
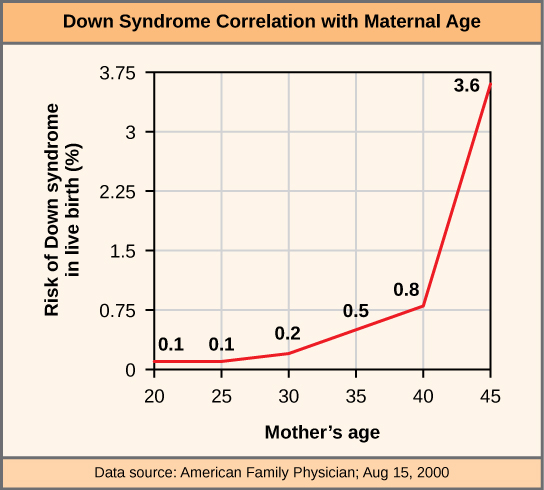
The incidence of Down syndrome is correlated with maternal age, such that older women are more likely to give birth to children with Down syndrome (Figure 4).
An individual with more than the correct number of chromosome sets (two for diploid species) is called polyploid. For instance, fertilization of an abnormal diploid egg with a normal haploid sperm would yield a triploid zygote. Polyploid animals are extremely rare, with only a few examples among the flatworms, crustaceans, amphibians, fish, and lizards. Triploid animals are sterile because meiosis cannot proceed normally with an odd number of chromosome sets. In contrast, polyploidy is very common in the plant kingdom, and polyploid plants tend to be larger and more robust than euploids of their species (Figure 5).

Sex Chromosome Nondisjunction
Humans display dramatic deleterious effects with autosomal trisomies and monosomies. Therefore, it may seem counterintuitive that human females and males can function normally, despite carrying different numbers of the X chromosome. In part, this occurs because of a process called X inactivation. Early in development, when female mammalian embryos consist of just a few thousand cells, one X chromosome in each cell inactivates by condensing into a structure called a Barr body. The genes on the inactive X chromosome are not expressed. The particular X chromosome (maternally or paternally derived) that is inactivated in each cell is random, but once the inactivation occurs, all cells descended from that cell will have the same inactive X chromosome. By this process, females compensate for their double genetic dose of X chromosome.
In so-called “tortoiseshell” cats, X inactivation is observed as coat-color variegation (Figure 6). Females heterozygous for an X-linked coat color gene will express one of two different coat colors over different regions of their body, corresponding to whichever X chromosome is inactivated in the embryonic cell progenitor of that region. When you see a tortoiseshell cat, you will know that it has to genetically be a female.

In an individual carrying an abnormal number of X chromosomes, cellular mechanisms will inactivate all but one X in each of her cells. As a result, X-chromosomal abnormalities are typically associated with mild mental and physical defects, as well as sterility. If the X chromosome is absent altogether, the individual will not develop.
Several errors in sex chromosome number have been characterized. Individuals with three X chromosomes, called triplo-X, appear female but express developmental delays and reduced fertility. The XXY chromosome complement, corresponding to one type of Klinefelter syndrome, corresponds to male individuals with small testes, enlarged breasts, and reduced body hair. The extra X chromosome undergoes inactivation to compensate for the excess genetic dosage. Turner syndrome, characterized as an X0 chromosome complement (i.e., only a single sex chromosome), corresponds to a female individual with short stature, webbed skin in the neck region, hearing and cardiac impairments, and sterility.
Chromosome Structural Rearrangements
Cytologists have characterized numerous structural rearrangements in chromosomes, including partial duplications, deletions, inversions, and translocations. Duplications and deletions often produce offspring that survive but exhibit physical and mental abnormalities. Cri-du-chat (from the French for “cry of the cat”) is a syndrome associated with nervous system abnormalities and identifiable physical features that results from a deletion of most of the small arm of chromosome 5 (Figure 7). Infants with this genotype emit a characteristic high-pitched cry upon which the disorder’s name is based.

Chromosome inversions and translocations can be identified by observing cells during meiosis because homologous chromosomes with a rearrangement in one of the pair must contort to maintain appropriate gene alignment and pair effectively during prophase I.
A chromosome inversion is the detachment, 180° rotation, and reinsertion of part of a chromosome (Figure 8). Unless they disrupt a gene sequence, inversions only change the orientation of genes and are likely to have more mild effects than aneuploid errors.
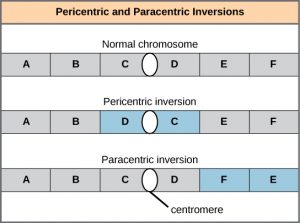
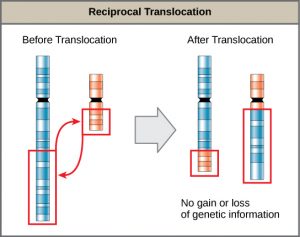
A translocation occurs when a segment of a chromosome dissociates and reattaches to a different, nonhomologous chromosome. Translocations can be benign or have devastating effects, depending on how the positions of genes are altered with respect to regulatory sequences. Notably, specific translocations have been associated with several cancers and with schizophrenia. Reciprocal translocations result from the exchange of chromosome segments between two nonhomologous chromosomes such that there is no gain or loss of genetic information (Figure 9).
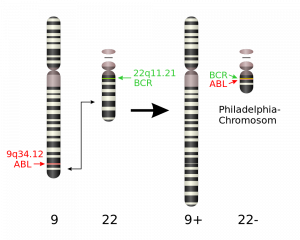
One specific example of a chromosomal translocation – the “Philadelphia chromosome” – is found in people who suffer from chronic myeloid leukemia (CML). In this translocation, a piece of chromosome 9 is swapped with a section of chromosome 22. This connects two genes on chromosome 22; one that was originally from chromosome 9 and one that was from chromosome 22. This translocation produces the BCR-ABL fusion protein, which causes white blood cells to divide out of control. BCR-ABL positive cancers can be treated with the drug Gleevac.
References
Unless otherwise noted, images on this page are licensed under CC-BY 4.0 by OpenStax.
OpenStax, Biology. OpenStax CNX. May 27, 2016 http://cnx.org/contents/s8Hh0oOc@9.10:6-3MVU-j@4/Errors-in-Meiosis

