92 Cotton Kills
Conduction
Cotton is a great thermal insulator – as long as it’s dry. Once wet, cotton becomes a poor insulator and does a poor job of preventing hypothermia-hence the old adage, “cotton kills”. To understand why cotton is sometimes good insulator and sometimes a poor one, we need to understand conduction, which is the transfer of thermal energy between objects by direct contact. The average kinetic energy of the molecules in a hot body is higher than in a colder body. If two molecules collide, an energy transfer from the molecule with greater kinetic energy to the molecule with less kinetic energy occurs. The cumulative effect from many collisions occurring at a surface of contact between the two objects results in transfer of heat from the hot object to the colder object, (or colder environment) in agreement with the Second Law of Thermodynamics.
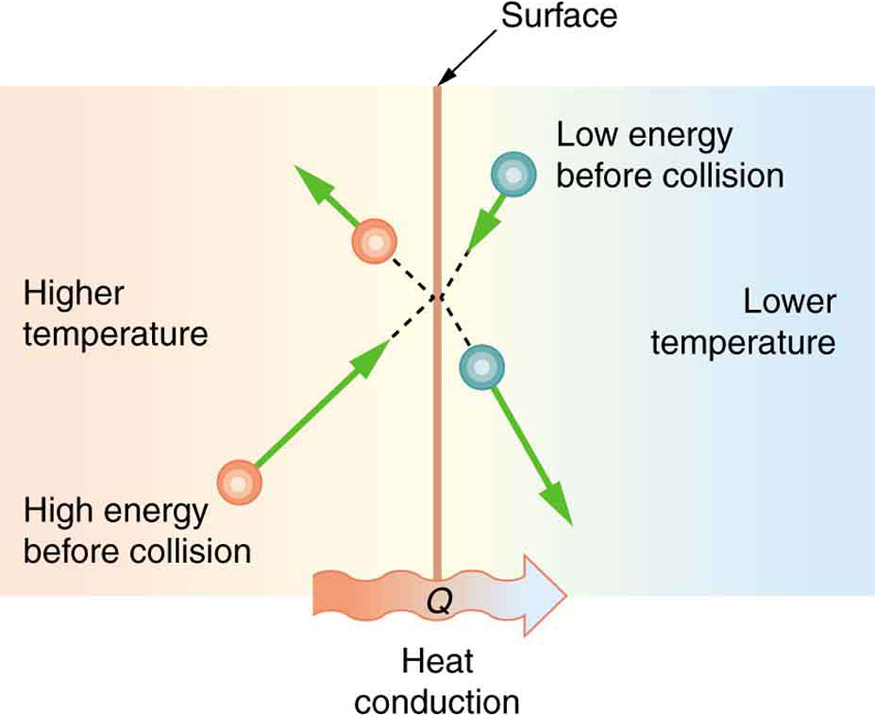
Contact Area
The number of molecular collisions increases with increasing contact area (![]() ), so the rate of heat conduction depends on the contact area. More collisions, and thus more time, will be needed to transfer the same amount of heat across a thicker material, so heat transfer rate also depends on thickness, or length across which the heat is transferred (
), so the rate of heat conduction depends on the contact area. More collisions, and thus more time, will be needed to transfer the same amount of heat across a thicker material, so heat transfer rate also depends on thickness, or length across which the heat is transferred (![]() ). This model explains why thick clothing is warmer than thin clothing in winters, and why thin people are typically more susceptible to hypothermia.
). This model explains why thick clothing is warmer than thin clothing in winters, and why thin people are typically more susceptible to hypothermia.
Temperature Difference
If the object temperatures are the same, the net heat transfer rate falls to zero, and thermal equilibrium is achieved. As the difference in temperature increases, the average kinetic energy transferred from fast to slow molecules during each collision also increases. Therefore the temperature difference also affects the rate of heat transfer by conduction.
Thermal Conductivity
Lastly, some materials conduct thermal energy faster than others. In general, good conductors of electricity (metals like copper, aluminum, gold, and silver) are also good heat conductors, whereas insulators of electricity (wood, plastic, and rubber) are poor heat conductors. The effect of a material’s properties on conductive heat transfer rate is described by the coefficient of thermal conductivity (![]() ), which is sometimes shortened to just thermal conductivity. The following table shows values of
), which is sometimes shortened to just thermal conductivity. The following table shows values of ![]() for some common materials in units of Watts, per meter, per Kelvin (
for some common materials in units of Watts, per meter, per Kelvin (![]() ).
).
| Substance | Thermal conductivity ( |
|---|---|
| Silver | 420 |
| Copper | 390 |
| Gold | 318 |
| Aluminum | 220 |
| Steel iron | 80 |
| Steel (stainless) | 14 |
| Ice | 2.2 |
| Glass (average) | 0.84 |
| Concrete brick | 0.84 |
| Water | 0.6 |
| Fatty tissue (without blood) | 0.2 |
| Asbestos | 0.16 |
| Plasterboard | 0.16 |
| Wood | 0.08–0.16 |
| Snow (dry) | 0.10 |
| Cork | 0.042 |
| Glass wool | 0.042 |
| Wool | 0.04 |
| Down feathers | 0.025 |
| Air | 0.023 |
| Styrofoam | 0.010 |
Conduction Equation
We can summarize all of the factors effecting the conductive heat transfer rate, which is an amount of energy transferred per time, in a single diagram:
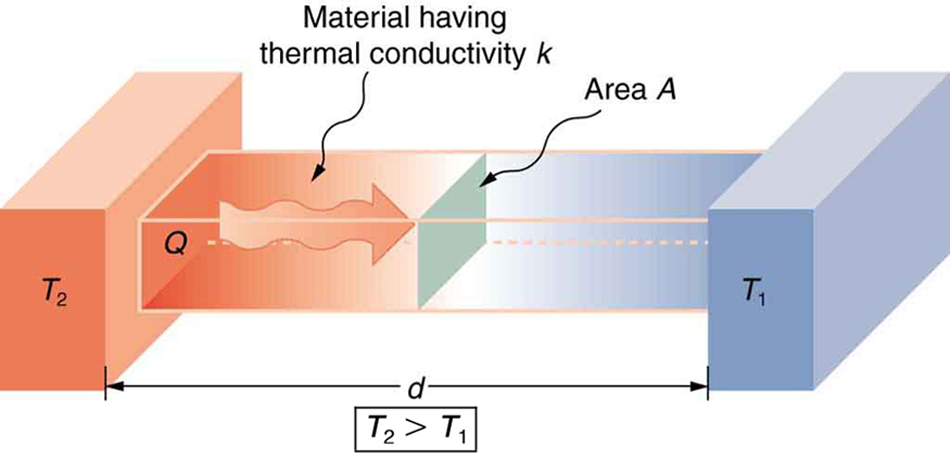
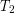 on the left and
on the left and 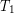 on the right, where
on the right, where 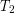 is greater than
is greater than 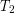 . The rate of heat transfer by conduction is directly proportional to the surface area A, the temperature difference, and the substance’s conductivity k. The rate of heat transfer is inversely proportional to the thickness d.
. The rate of heat transfer by conduction is directly proportional to the surface area A, the temperature difference, and the substance’s conductivity k. The rate of heat transfer is inversely proportional to the thickness d.The rate of heat transfer across a material with temperature ![]() on one side and temperature
on one side and temperature ![]() on the other can be modeled by the conduction equation:
on the other can be modeled by the conduction equation:
(1) ![]()
The rate of heat transfer (![]() )refers to an amount of energy transferred per unit time so it has the same units as power (
)refers to an amount of energy transferred per unit time so it has the same units as power (![]() ). The term power is usually applied to a rate of transformation of energy from one form to another, but heat is a transfer of thermal energy from one place another, not a change in type of energy, thus we stick with
). The term power is usually applied to a rate of transformation of energy from one form to another, but heat is a transfer of thermal energy from one place another, not a change in type of energy, thus we stick with ![]() instead of
instead of ![]() as the notation for this rate. This notation also reminds us that if we want to know how much energy was transferred during a certain time, we need to multiply the heat rate by the time.
as the notation for this rate. This notation also reminds us that if we want to know how much energy was transferred during a certain time, we need to multiply the heat rate by the time.
Insulation Technology
Everyday Example: Down Insulation
Let’s compare the insulating properties of a cotton sweatshirt and a down jacket, such as the one worn in the photograph below.

We will start with the conduction equation:
![]()
The outside temperature was about ![]() and skin temperature is roughly
and skin temperature is roughly ![]() , so the temperature difference was about
, so the temperature difference was about ![]() . When dry, the down jacket pictured is roughly
. When dry, the down jacket pictured is roughly ![]() thick, which
thick, which ![]() . The thermal conductivity of dry down is roughly
. The thermal conductivity of dry down is roughly ![]() . Using the methods in Chapter 17 we estimate the surface area of the upper body to be
. Using the methods in Chapter 17 we estimate the surface area of the upper body to be ![]() . Entering these values into the equation we find:
. Entering these values into the equation we find:
![]()
So heat loss rate through the upper body in this situation is 30 W. That means 15 J of thermal energy is released to the environment as heat through the upper body each second. The typical human thermal power is 100 W so it appears that there is plenty of thermal energy available to maintain temperature. However, the 15 W value we found does not account for heat loss from the lower body, which we can see from the picture were not covered with down. In fact the single thin layer worn on the legs easily allowed for the other 85 W to escape so that body temperature was not elevated. In fact, doing the extra work of climbing the mountain likely raised the the thermal power closer to 300 W so actually something closer to 285 W was being exhausted through the lower body. As a result, body temperature dropped quickly when climbing stopped and the thermal power fell back to about 100 W.
If the down were to get wet, things would change drastically. The down would lose its loft and end up with roughly 0.5 cm thickness. Even worse, the water would fill in the air spaces between the down fibers so the thermal conductivity would essentially be the same as for water, which is ![]() . Entering these values into the conduction equation we find:
. Entering these values into the conduction equation we find:
![]()
Now that’s a problem. The wet heat loss rate is nearly 190x faster than for the dry down in this situation, so to keep up we would need to eat 188 candy bars every six hours, or 31 candy bars per hour! Our bodies would not be able to digest and convert chemical potential energy to thermal energy fast enough to keep warm in that situation and eventually hypothermia would occur. Later in this unit we will be able to estimate how long it would take for the body temperature to drop to dangerous levels in this situation. Down is clearly a poor insulator when wet, but even after drying the fibers do not naturally recover their original loft. Down is a poor choice of insulation in wet environments, though it does well in snow storms as long as the air temperature is cool enough that the snow doesn’t melt when it lands on the jacket.
Everyday Examples: Cotton Kills
Just as with down, water can permeate the spaces between other fabrics like wool, synthetics, and cotton. The majority of water can be wrung out of wool and synthetics, partially restoring their insulating properties and helping them to dry out quickly and recover full insulation value. In the other hand, water fills space between cotton fibers and also saturates the fibers themselves. As a result cotton does not wring out well and dries slowly, so its thermal conductivity remains much closer to that of water than wool or synthetic. Cotton is a poor choice of insulation in wet environments.
Reinforcement Exercise: Single Pane Windows
Significant content in this chapter, including the table of conductivities, was adapted from College Physics, by BC Open Textbooks [1]
- "Conduction" by College Physics, BC Open Textbooks is licensed under CC BY 4.0 ↵
The condition of having a body temperature well below the normal range.
the process by which heat or directly transmitted through a substance when there is a difference of temperature between adjoining regions, without movement of the material
energy stored in the microscopic motion of atoms and molecules (microscopic kinetic energy)
energy which a body possesses by virtue of being in motion, energy stored by an object in motion
the total entropy of an isolated system can never decrease over time, meaning objects left to themselves will always trend toward thermal equilibrium, meaning that thermal energy will always spontaneously transfer from hot system to cold system
An amount of thermal energy transferred due to a difference in temperature.
a two systems are in thermal equilibrium when they do not exchange heat, which means they must be at the same temperature
a measure of a material's ability to conduct heat
international standard unit of power, equal to one Joule per second
SI unit of temperature
amount of thermal energy transferred into our, out of an object as heat, per unit time
the rate at which work is done, the rate at which energy is converted from one form to another
materials designed to slow the rate of heat transfer
a measure of the average kinetic energy of the particles (e.g., atoms and molecules) in an object, which determines how relatively hot or cold an object feels
the amount of heat (thermal energy transferred due to a temperature difference) that leaves an object per unit time
rate at which chemical potential energy is converted to thermal energy by the body, batteries, or heat engines. Also, rate at which thermal energy is converted to electrical energy by a thermal power plant.
A quantity representing the effect of applying a force to an object or system while it moves some distance.
energy stored in the chemical bonds of a substance

